Abstract
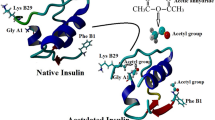
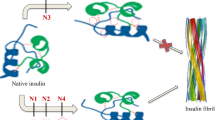
Homocysteine thiolactone (HCTL) is a cyclic thioester of homocysteine, showing high reactivity toward lysine residues of proteins. In the present study the structural properties and aggregation propensity of bovine pancreatic insulin were studied in the presences of increasing concentration of HCTL (0–500 μM), using different spectroscopic techniques. As shown in this study, HCTL induces gross structural alterations and subsequently aggregation of insulin in a dose dependent manner. Also induction of insulin aggregation by HCTL occurs in a sequential process, where native protein with alpha-helical abundant structure gradually transforms into partially folded conformations with the significant amount of beta-sheet. Since C-terminal B-chain of insulin plays a critical role in stability of this protein, the structural alteration/aggregation induced by HCTL can be consequence of homocysteinylation of the only Lysine residue (Lys29) on its B-chain. This study may have important implications regarding the effect of HCTL on structure of insulin particularly in the pathological states linked to hyperhomocysteinemia.





Similar content being viewed by others
Abbreviations
- ANS:
-
1-anilino-8-naphthalene sulfonate
- BLH:
-
Bleomycin hydrolase
- CBS:
-
Cystathionine β-synthase
- CD:
-
Circular dichroism
- CDNN:
-
Context dependent neural networks
- DLS:
-
Dynamic light scattering
- HCTL:
-
Homocysteine thiolactone
- Hcy:
-
Homocysteine
- Lys:
-
Lysine
- MetRS:
-
Methionyl-tRNA synthetase
- MS:
-
Met synthase
- MTHFR:
-
Methylene tetrahydrofolate reductase
- PON1:
-
Paraoxonase 1
- RH :
-
Hydrodynamic radius
- UF:
-
Ultra filtration
References
Ahmad A, Uversky VN, Hong D, Fink AL (2005) J Biol Chem 280:42669–42675
Baillie SE, Clegg W, García-Álvarez P, Hevia E, Kennedy AR, Klett J, Russo L (2011) Chem Commun (Camb) 47:388–390
Bełtowski J (2005) Postepy Hig Med Dosw 59:392–404
Böhm G, Muhr R, Jaenicke R (1992) Protein Eng 5:191–195
Bostom AG, Lathrop L (1997) Kidney Int 52:10–20
Bouchard M, Zurdo J, Nettleton EJ, Dobson CM, Robinson CV (2000) Protein Sci 9:1960–1967
Brange J, Langkjoer L (1993) Pharm Biotechnol 5:315–350
Brosnan JT, Jacobs RL, Stead LM, Brosnan ME (2004) Acta Biochim Pol 51:405–413
Casas-Finet JR, Karpel RL, Maki AH, Kumar A, Wilson SH (1991) J Mol Biol 221:693–709
Chwatko G, Boers GH, Strauss KA, Shih DM, Jakubowski H (2007) FASEB J 21:1707–1713
D’Angelo A, Selhub J (1997) Blood 90:1–11
Demeule B, Lawrence MJ, Drake AF, Gurny R, Arvinte T (2007) Biochim Biophys Acta 1774:146–153
Dennis VW, Robinson K (1996) Kidney Int Suppl 57:11–17
Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB (1988) Diabetologia 31:158–161
Divsalar A, Saboury AA, Moosavi-Movahedi AA (2006) Protein J 25:157–165
Domagała TB, Łacinski M, Trzeciak WH, Mackness B, Mackness MI, Jakubowski H (2006) Cell Mol Biol (Noisy-le-grand) 52:4–10
Green R, Jacobsen DW (1995) In: Bailey LB (ed) Folate in health and disease. Marcel Dekker, New York, pp 75–122
Hawe A, Sutter M, Jiskoot W (2008) Pharm Res 25:1487–1499
Home P, Bartley P, Russell-Jones D, Hanaire-Broutin H, Heeg JE, Abrams P, Landin-Olsson M, Hylleberg B, Lang H, Draeger E (2004) Diabetes Care 27:1081–1087
Hong DP, Ahmad A, Fink AL (2006) Biochemistry 45:9342–9353
Jacobsen DW (1998) Clin Chem 44:1833–1843
Jakubowski H (1991) EMBO J 10:593–598
Jakubowski H (1997) J Biol Chem 272:1935–1942
Jakubowski H (1999) FASEB J 13:2277–2283
Jakubowski H (2000) J Biol Chem 275:3957–3962
Jakubowski H (2001) Biomed Pharmacother 55:443–447
Jakubowski H (2002) Anal Biochem 308:112–119
Jakubowski H (2002) J Biol Chem 277:30425–30428
Jakubowski H (2003) Clin Chem Lab Med 41:1462–1466
Jakubowski H (2004) Mol Cell Life Sci 61:470–487
Jakubowski H (2006) J Nutr 136:1741–1749
Jakubowski H (2007) Clin Chem Lab Med 45:1704–1716
Jakubowski H (2008) J Physiol Pharmacol 59:155–167
Jakubowski H (2010) Adv Exp Med Biol 660:113–127
Jakubowski H, Ambrosius WT, Pratt JH (2001) FEBS Lett 491:35–39
Jiménez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR (2002) Proc Natl Acad Sci 99:9196–9201
Kim HY, Ghosh G, Schulman LH, Brunie S, Jakubowski H (1993) Proc Natl Acad Sci 90:11553–11557
Knekt P, Reunanen A, Alfthan G, Heliövaara M, Rissanen H, Marniemi J, Aromaa A (2001) Arch Intern Med 161:1589–1594
Krajcovicová-Kudlácková M, Blazícek P, Ginter E, Valachicová M (2004) Cent Eur J Public Health 12:217–219
Lasagna-Reeves CA, Clos AL, Midoro-Hiriuti T, Goldblum RM, Jackson GR, Kayed R (2010) Endocrinology 151:4717–4724
Mansoor MA, Ueland PM, Aarsland A, Svardal AM (1993) Metabolism 42:1481–1485
Mayer EL, Jacobsen DW (1996) J Am Coll Cardiol 27:517–527
Menendez CJ, Herskovits TT (1969) Biochemistry 8:5052–5059
Mercié P, Garnier O, Lascoste L, Renard M, Closse C, Durrieu F, Marit G, Boisseau RM, Belloc F (2000) Apoptosis 5:403–411
Mudd SH, Levy HL, Krauss JP (2001) In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B (eds) The metabolic and molecular bases of inherited disease. C MC Graw Hill, New York, pp 2007–2056
Mudd SH, Levy HL, Skovby F (1995) In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited disease. McGraw Hill, New York, pp 1279–1327
Najib S, Sánchez-Margalet V (2001) J Mol Endocrinol 27:85–91
Ndrepepa G, Kastrati A, Braun S, Koch W, Kölling K, Mehilli J, Schömig A (2008) Metab Cardiovasc Dis 18:66–73
Nielsen L, Frokjaer S, Brange J, Uversky VN, Fink AL (2001) Biochemistry 40:8397–8409
Pancharuniti N, Lewis CA, Sauberlich EH, Perkins LL, Go PCG, Alvarez JO, Macaluso M, Acton RT, Copeland RB, Cousins AL (1994) Am J Clin Nutr 59:940–948
Panza G, Dumpitak C, Birkmann E (2010) Rejuvenation Res 13:220–223
Perdziak M, Zimny J, Jakubowski H, Guranowski A (2005) Acta Biochim Polon 50 (Abstract P11.49)
Pocker Y, Biswas SB (1980) Biochemistry 19:5043–5049
Pocker Y, Biswas SB (1981) Biochemistry 20:4354–4361
Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ (1998) JAMA 279:359–364
Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer F (2004) J Biol Chem 279:14844–14852
Sauls DL, Wolberg AS, Hoffman M (2003) J Thromb Haemost 1:300–306
Selkoe DJ (2003) Nature 426:900–904
Sen U, Tyagi SC (2010) PPAR Res 2010:1–12
Stefani M, Dobson CM (2003) J Mol Med 81:678–699
Ueland PM, Refsum H, Brattström L (1992) In: Francis RB (ed) Atherosclerotic cardiovascular disease, hemostasis and endothelial function. Marcel Dekker, New York, pp 183–236
Wallace BA (2009) Q Rev Biophys 42:317–370
Wang R, Zenobi R (2010) J Am Soc Mass Spectrom 21:378–385
Wang SS, Liu KN, Han TC (2010) Biochim Biophys Acta 1802:519–530
Wright CB, Lee HS, Paik MC, Stabler SP, Allen RH, Sacco RL (2004) Neurology 63:254–260
Yang S, Levine H, Onuchic JN (2005) J Mol Biol 352:202–211
Yong Z, Yingjie D, Ming L, Craig DQ, Zhengqiang L (2009) J Colloid Interface Sci 337:322–331
Yousefi R, Ardestani SK, Saboury AA, Kariminia A, Zeinali M, Amani M (2005) J Biochem Mol Biol 38:407–413
Zimny J, Sikora M, Guranowski A, Jakubowski H (2006) J Biol Chem 2481:22485–22492
Acknowledgments
We would like to thank the research council of Shiraz University for the financial support (Grant number: 88-GR-SCST-112). Also the support of Iran National Science Foundation (INSF)/Grant number: 88001578 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalili, S., Yousefi, R., Papari, MM. et al. Effect of Homocysteine Thiolactone on Structure and Aggregation Propensity of Bovine Pancreatic Insulin. Protein J 30, 299–307 (2011). https://doi.org/10.1007/s10930-011-9333-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9333-1