Abstract
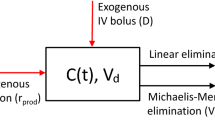
The main objective of this article is to propose the closed-form solution of one-compartment pharmacokinetic model with simultaneous first-order and Michaelis–Menten elimination for the case of constant infusion. For the case of bolus administration, we have previously established a closed-form solution of the model through introducing a transcendent X function. In the same vein, we found here a closed-form solution of constant infusion could be realized through introducing another transcendent Y function. For the general case of constant infusion of limited duration, the closed-form solution is then fully expressed using both X and Y functions. As direct results, several important pharmacokinetic surrogates, such as peak concentration \(C_{max}\) and total drug exposure AUC\(_{0-\infty }\), are found the closed-form expressions and ready to be analyzed. The new pharmacokinetic knowledge we have gained on these parameters, which largely exhibits in a nonlinear feature, is in clear contrast to that of the linear case. Finally, with a pharmacokinetic model adapted from that formerly reported on phenytoin, we numerically analyzed and illustrated the roles of different model parameters and discussed their influence on drug exposure. To conclude, the present findings elucidate the intrinsic quantitative structural properties of such pharmacokinetic model and provide a new avenue for future modelling and rational drug designs.




Similar content being viewed by others
References
Shi S (2014) Biologics: an update and challenge of their pharmacokinetics. Curr Drug Metab 15(3):271–290
Klitgaard T, Nielsen JN, Skettrup MP, Harper A, Lange M (2009) Population pharmacokinetic model for human growth hormone in adult patients in chronic dialysis compared with healthy subjects. Growth Horm IGF Res 19(6):463–470
Jin F, Krzyzanski W (2004) Pharmacokinetic model of target-mediated disposition of thrombopoietin. AAPS Pharm Sci 6(1):E9
Foley C, Mackey MC (2009) Mathematical model for G-CSF administration after chemotherapy. J Theor Biol 257:27–44
Frymoyer A, Juul SE, Massaro AN, Bammler TK, Wu YW (2017) High-dose erythropoietin population pharmacokinetics in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Pediatr Res 81(6):865–872
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49(10):633–659
Kozawa S, Yukawa N, Liu J, Shimamoto A, Kakizaki E, Fujimiya T (2007) Effect of chronic ethanol administration on disposition of ethanol and its metabolites in rat. Alcohol 41(2):87–93
Craig M, Humphries AR, Nekka F, Bélair J, Li J, Mackey MC (2014) Neutrophil dynamics during concurrent chemotherapy and G-CSF administration: mathematical modelling guides dose optimisation to minimise neutropenia. J Theor Biol 385:77–89
Scholz M, Schirm S, Wetzler M, Engel C, Loeffler M (2012) Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans. Theor Biol Med Model 9:32
Woo S, Krzyzanski W, Jusko WJ (2006) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther 319(3):1297–306
Tang S, Xiao Y (2007) One-compartment model with Michaelis–Menten elimination kinetics and therapeutic window: an analytical approach. J Pharmacokinet Pharmacodyn 34:807–827
Beal SL (1982) On the solution to the Michaelis–Menten equation. J Pharmacokinet Biopharm 10:109–119
Wilkinson PK, Sedman AJ, Sakmar E, Kay DR, Wagner JG (1977) Pharmacokinetics of ethanol after oral administration in the fasting state. J Pharmacokinet Biopharm 5(3):207–224
Wu X, Li J, Nekka F (2015) Closed form solutions and dominant elimination pathways of simultaneous first-order and Michaelis–Menten kinetics. J Pharmacokinet Pharmacodyn 42:151–161
Wu X, Nekka F, Li J (2018) Mathematical analysis and drug exposure evaluation of pharmacokinetic models with endogenous production and simultaneous first-order and Michaelis–Menten elimination: The case of single dose. J Pharmacokinet Pharmacodyn 45(5):693–705
Wu X, Nekka F, Li J (2019) Analytical solution and exposure analysis of a pharmacokinetic model with simultaneous elimination pathways and endogenous production: The case of multiple dosing administration. Bull Math Biol 81(9):3436–3459
Paul M, Ram R, Kugler E et al (2014) Subcutaneous versus intravenous granulocyte colony stimulating factor for the treatment of neutropenia in hospitalized hemato-oncological patients: randomized controlled trial. Am J Hematol 89(3):243–248
Saelue P, Sripakdee W, Suknuntha K (2019) Effects of drug concentration, rate of infusion, and flush volume on G-CSF drug loss when administered intravenously. Hosp Pharm. 54(6):393–397
Luo S, McSweeney KM, Wang T, Bacot SM, Feldman GM, Zhang B (2020) Defining the right diluent for intravenous infusion of therapeutic antibodies. MAbs. 12(1):1685814
Evans WS, Anderson SM, Hull LT, Azimi PP, Bowers CY, Veldhuis JD (2001) Continuous 24-hour intravenous infusion of recombinant human growth hormone (GH)-releasing hormone-(1–44)-amide augments pulsatile, entropic, and daily rhythmic GH secretion in postmenopausal women equally in the estrogen-withdrawn and estrogen-supplemented states. J Clin Endocrinol Metab 86(2):700–712
Corless RM, Gonnet GH, Hare DEG, Jeffrey DJ, Knuth DE (1996) On the Lambert W function. Adv Comput Math 5:329–359
Wu X, Li J (2021) Morphism classification of a family of transcendent functions arising from pharmacokinetic modelling. Math Meth Appl Sci. 44(1):140–152
Jusko WJ, Koup JR, Alván G (1976) Nonlinear assessment of phenytoin bioavailability. J Pharmacokinet Biopharm 4(4):327–336
Acknowledgements
The authors would like to thank the financial support from National Natural Science Foundation of China (Grant No. 12071300), Natural Sciences and Engineering Research Council of Canada, and Le Fonds de recherche du Québec-Nature et technologies (FRQNT). We would also like to thank two anonymous reviewers for their helpful and insightful comments which leads to the high improvement of the article’s quality.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Proof of Lemma 1
Proof
(i) It is clear that \(C^{\infty }\) is the unique positive equilibrium of Model (8) since \(\frac{dC(t)}{dt}\bigg |_{C(t)=C^{\infty }}=0\). Moreover, we have \(C'(t)>0\) as long as \(0\le C(t)< C^{\infty }\), resulting in C(t) is monotonically increasing as t increases. Hence, \(C^{\infty }\) is an upper bound for C(t) for \(t>0\). As well, when \(0\le C(t)<C^{\infty }\), the second order derivative
implying \(C'(t)\) decreases to zero as \(t\rightarrow \infty \). By the monotone bounded convergence theorem, we can deduce that \(C^{\infty }\) is the upper limit for C(t).
(ii) Denote a function by \(f(x)=\sqrt{x^2+a}+x\,(a>0)\) for a real variable x. It follows \(f'(x)=x/\sqrt{x^2+a}+1>0\) that f(x) is strictly increasing with respect to x. Let \(a= 4\frac{r}{k_{el}}K_m>0\), then we obtain
since \(C_{\beta }>K_m\)□.
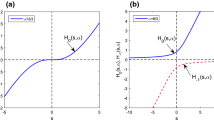
Appendix 2: Illustration of \(X_0\) and \(Y_0\) for different values of \(p_1\), \(p_2\), \(q_1\) and \(q_2\)
In Fig. 5, we plot how parameters \(p_1\), \(p_2\), \(q_1\) and \(q_2\) change the appearance of X and Y functions in the first quadrant, where \(p_1+p_2=1\) and \(q_1+q_2=1\) are considered. As shown in Fig. 5a, when \(p_1\) varies from a small value of 1/500 to a large value of 1/2, we observe that \(X_0(s,p_1,p_2)\) tends to increase faster for a larger value of \(p_1\), and when \(p_1\) is close to unity, \(X_0(s,p_1,p_2)\) is close to the identity line as \(X_0(s,p_1,p_2)=s\). In Fig. 5b, \(Y_0(s,q_1,q_2)\) shows a similar property as a faster increase of \(Y_0(s,q_1,q_2)\) can be observed for a larger \(q_1\). Meanwhile, the \(Y(s,q_1,q_2)\) is also close to the identity line as \(Y_0(s,q_1,q_2)=s\) when \(q_1\) is close to unity. The range of \(Y_0(s,q_1,q_2)\) is \((0,q_1/(q_1+q_2))\) that depends on the choice of \(q_1\) and \(q_2\).
Illustration of how parameters \(p_1\), \(p_2\), \(q_1\) and \(q_2\) affect the graphs of principal real branches \(X_0\) and \(Y_0\) transcendent functions in the first quadrant, where \(p_1+p_2=q_1+q_2=1\), \(X_0\in (0,\infty )\) for all \(s>0\) and \(Y_0\in (0,q_1)\subset (0,1)\) for all \(s\in (0,q_1^{q_1}q_2^{q_2})\subset (0,1)\)
Appendix 3: Proof of Proposition 1
Proof
(i) By checking the explicit expressions of \(C^{\infty }\) and \(C^{\infty }_{\beta }\), it is easy to see \(C^{\infty }=0\) and \(C^{\infty }_{\beta }=C_{\beta }\) when \(r=0\).
(ii) If \(\displaystyle C_{\beta }=\frac{r}{k_{el}}\), namely \(r=k_{el}C_{\beta }=k_{e,tot}K_m\), then we have \(\displaystyle C^{\infty }=C^{\infty }_{\beta }=\sqrt{\frac{k_{e,tot}}{k_{el}}}K_m\). If \(\displaystyle C_{\beta }>\frac{r}{k_{el}}\), namely \(r<k_{el}C_{\beta }\), then we obtain \(C^{\infty }_{\beta }>C^{\infty }\). If \(\displaystyle C_{\beta }<\frac{r}{k_{el}}\), we have \(r<k_{el}C_{\beta }\) and \(C^{\infty }_{\beta }<C^{\infty }\).
(iii) Consider the derivatives of \(C^{\infty }\) and \(C^{\infty }_{\beta }\) with respect to variable r. With straightforward calculations and denote \(\displaystyle x=\frac{r}{k_{el}}\), we obtain
and
due to \(K_m<C^{\infty }_{\beta }\) by Lemma 1. Therefore with respect to r, \(C^{\infty }\) is an increasing function and \(C^{\infty }_{\beta }\) is a decreasing function.
Now we consider the limit of \(C^{\infty }_{\beta }\) as r tends to infinity. Multiplying by
at both the numerator and denominator for expression of \(C^{\infty }_{\beta }\) if we write \(C^{\infty }_{\beta }\) as \(C^{\infty }_{\beta }/1\), we obtain
\(\displaystyle \lim _{r\rightarrow +\infty }C^{\infty }=+\infty \) is obvious.□
Appendix 4: Explicit solutions of one-compartment models with a single elimination pathway, linear or Michaelis–Menten, in the case of constant infusion
One-compartment pharmacokinetic model with a single linear elimination pathway for a constant infusion:
where f(t) is given by Eq. (18). Its explicit solution is
One-compartment pharmacokinetic model with a single Michaelis–Menten elimination pathway for a constant infusion:
where f(t) is given by Eq. (18). Its explicit solution is
(i) If \(0\le t\le T\), we have
(ii) If \(t\ge T\), we have
Rights and permissions
About this article
Cite this article
Wu, X., Chen, M. & Li, J. Constant infusion case of one compartment pharmacokinetic model with simultaneous first-order and Michaelis–Menten elimination: analytical solution and drug exposure formula. J Pharmacokinet Pharmacodyn 48, 495–508 (2021). https://doi.org/10.1007/s10928-021-09740-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-021-09740-5