Abstract
Regulatory agencies have a strong interest in sensitivity analysis for the evaluation of physiologically-based pharmacokinetic (PBPK) models used in pharmaceutical research and drug development and regulatory submissions. One of the applications of PBPK is the prediction of fraction absorbed and bioavailability for drugs following oral administration. In this context, we performed a variance based global sensitivity analysis (GSA) on in-house PBPK models for drug absorption, with the aim of identifying key parameters that influence the predictions of the fraction absorbed and the bioavailability for neutral, acidic and basic compounds. This analysis was done for four different classes of drugs, defined according to the Biopharmaceutics Classification System, differentiating compounds by permeability and solubility. For class I compounds (highly permeable, highly soluble), the parameters that mainly influence the fraction absorbed are related to the formulation properties, for class II compounds (highly permeable, lowly soluble) to the dissolution process, for class III (lowly permeable, highly soluble) to both absorption process and formulation properties and for class IV (lowly permeable, lowly soluble) to both absorption and dissolution processes. Considering the bioavailability, the results are similar to those for the fraction absorbed, with the addition that parameters related to gut wall and liver clearance influence as well the predictions. This work aimed to give a demonstration of the GSA methodology and highlight its importance in improving our understanding of PBPK absorption models and in guiding the choice of parameters that can safely be assumed, estimated or require data generation to allow informed model prediction.










Similar content being viewed by others
Notes
Let us consider the model Y = X1 · X2, with X1 distributed normally with mean equal to 1 and variance equal to 1 and X2 distributed normally with mean equal to 0 and variance equal to 1. The main effect of X1 is equal to 0, because X2 has mean 0. Thus, by limiting the analysis on the main effect, one may conclude that X1 has no impact on V(Y). Intuitively, this conclusion is wrong. In fact, X1 impact can be observed if X2 is allowed to vary from its mean value. Thus, X1 impact on V(Y) is due to interaction effect with X2.
The codes used to perform the analysis are available at the following link: http://aimed11.unipv.it/JPKPDMelillo18/.
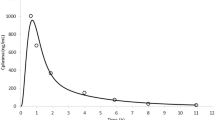
Atenolol is a BCS class III drug with a mean in vivo Peff of 0.5 × 10−4 cm/s and a standard deviation of 0.2 × 10−4 cm/s [5, 34]. Supposing that Peff is distributed log-normally, the 95th percentile is equal to 0.47 and the 5th percentile is below the inferior limit in Table 1. So, for Atenolol the range of variability of Peff, from the lower limit in Table 1 to its 95th percentile, represents around 30% of the whole range of variation considered for class III drugs.
References
Margolskee A, Darwich AS, Pepin X, Pathak SM, Bolger MB, Aarons L, Rostami-Hodjegan A, Angstenberger J, Graf F, Laplanche L, Müller T, Carlert S, Daga P, Murphy D, Tannergren C, Yasin M, Greschat-Schade S, Mück W, Muenster U, van der Mey D, Frank KJ, Lloyd R, Adriaenssen L, Bevernage J, De Zwart L, Swerts D, Tistaert C, Van Den Bergh A, Van Peer A, Beato S, Nguyen-Trung A-T, Bennett J, McAllister M, Wong M, Zane P, Ollier C, Vicat P, Kolhmann M, Marker A, Brun P, Mazuir F, Beilles S, Venczel M, Boulenc X, Loos P, Lennernäs H, Abrahamsson B (2017) IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success part 1: characterisation of the OrBiTo database of compounds. Eur J Pharm Sci 96:598–609. https://doi.org/10.1016/j.ejps.2016.09.027
Amidon GL, Lennernäs H, Shah VP, Crison JR (1995) A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–420. https://doi.org/10.1023/A:1016212804288
Dahan A, Miller JM, Amidon GL (2009) Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J 11:740–746. https://doi.org/10.1208/s12248-009-9144-x
CDER (FDA) (2000) Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a Biopharmaceutics Classification System. Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration (FDA), Rockville
Lennernäs H (2014) Human in vivo regional intestinal permeability: importance for pharmaceutical drug development. Mol Pharm 11:12–23. https://doi.org/10.1021/mp4003392
Agoram B, Woltosz WS, Bolger MB (2001) Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev 50:S41–S67. https://doi.org/10.1016/S0169-409X(01)00179-X
Cong D, Doherty M, Pang KS (2000) A new physiologically based, segregated-flow model to explain route-dependent intestinal metabolism. Drug Metab Dispos Biol Fate Chem 28:224–235
Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A (2010) Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab 11:716–729. https://doi.org/10.2174/138920010794328913
Gertz M, Houston JB, Galetin A (2011) Physiologically based pharmacokinetic modeling of intestinal first-pass metabolism of CYP3A substrates with high intestinal extraction. Drug Metab Dispos 39:1633–1642. https://doi.org/10.1124/dmd.111.039248
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, Tucker G (2009) Population-based mechanistic prediction of oral drug absorption. AAPS J 11:225–237. https://doi.org/10.1208/s12248-009-9099-y
Willmann S, Schmitt W, Keldenich J, Lippert J, Dressman JB (2004) A physiological model for the estimation of the fraction dose absorbed in humans. J Med Chem 47:4022–4031. https://doi.org/10.1021/jm030999b
Parrott N, Lave T (2008) Applications of physiologically based absorption models in drug discovery and development. Mol Pharm 5:760–775. https://doi.org/10.1021/mp8000155
Jamei M (2016) Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep 2:161–169. https://doi.org/10.1007/s40495-016-0059-9
Jones HM, Dickins M, Youdim K, Gosset JR, Attkins NJ, Hay TL, Gurrell IK, Logan YR, Bungay PJ, Jones BC, Gardner IB (2012) Application of PBPK modelling in drug discovery and development at Pfizer. Xenobiotica 42:94–106. https://doi.org/10.3109/00498254.2011.627477
Lennernäs H, Aarons L, Augustijns P, Beato S, Bolger M, Box K, Brewster M, Butler J, Dressman J, Holm R, Julia Frank K, Kendall R, Langguth P, Sydor J, Lindahl A, McAllister M, Muenster U, Müllertz A, Ojala K, Pepin X, Reppas C, Rostami-Hodjegan A, Verwei M, Weitschies W, Wilson C, Karlsson C, Abrahamsson B (2014) Oral biopharmaceutics tools—time for a new initiative—an introduction to the IMI project OrBiTo. Eur J Pharm Sci 57:292–299. https://doi.org/10.1016/j.ejps.2013.10.012
Darwich AS, Margolskee A, Pepin X, Aarons L, Galetin A, Rostami-Hodjegan A, Carlert S, Hammarberg M, Hilgendorf C, Johansson P, Karlsson E, Murphy D, Tannergren C, Thörn H, Yasin M, Mazuir F, Nicolas O, Ramusovic S, Xu C, Pathak SM, Korjamo T, Laru J, Malkki J, Pappinen S, Tuunainen J, Dressman J, Hansmann S, Kostewicz E, He H, Heimbach T, Wu F, Hoft C, Pang Y, Bolger MB, Huehn E, Lukacova V, Mullin JM, Szeto KX, Costales C, Lin J, McAllister M, Modi S, Rotter C, Varma M, Wong M, Mitra A, Bevernage J, Biewenga J, Van Peer A, Lloyd R, Shardlow C, Langguth P, Mishenzon I, Nguyen MA, Brown J, Lennernäs H, Abrahamsson B (2017) IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success part 3: identifying gaps in system parameters by analysing In Silico performance across different compound classes. Eur J Pharm Sci 96:626–642. https://doi.org/10.1016/j.ejps.2016.09.037
Margolskee A, Darwich AS, Pepin X, Aarons L, Galetin A, Rostami-Hodjegan A, Carlert S, Hammarberg M, Hilgendorf C, Johansson P, Karlsson E, Murphy D, Tannergren C, Thörn H, Yasin M, Mazuir F, Nicolas O, Ramusovic S, Xu C, Pathak SM, Korjamo T, Laru J, Malkki J, Pappinen S, Tuunainen J, Dressman J, Hansmann S, Kostewicz E, He H, Heimbach T, Wu F, Hoft C, Laplanche L, Pang Y, Bolger MB, Huehn E, Lukacova V, Mullin JM, Szeto KX, Costales C, Lin J, McAllister M, Modi S, Rotter C, Varma M, Wong M, Mitra A, Bevernage J, Biewenga J, Van Peer A, Lloyd R, Shardlow C, Langguth P, Mishenzon I, Nguyen MA, Brown J, Lennernäs H, Abrahamsson B (2017) IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success part 2: an introduction to the simulation exercise and overview of results. Eur J Pharm Sci 96:610–625. https://doi.org/10.1016/j.ejps.2016.10.036
Zhang X-Y, Trame M, Lesko L, Schmidt S (2015) Sobol sensitivity analysis: a tool to guide the development and evaluation of systems pharmacology models. CPT Pharmacomet Syst Pharmacol 4:69–79. https://doi.org/10.1002/psp4.6
Saltelli A, Tarantola S, Campolongo F, Ratto M (2004) Sensitivity analysis in practice: a guide to assessing scientific models. Wiley, San Francisco
Borgonovo E, Plischke E (2016) Sensitivity analysis: a review of recent advances. Eur J Oper Res 248:869–887. https://doi.org/10.1016/j.ejor.2015.06.032
Iooss B, Lemaître P (2015) A review on global sensitivity analysis methods. In: Meloni C, Dellino G (eds) Uncertainty management in simulation-optimization of complex systems: algorithms and applications. Springer, New York
Pianosi F, Beven K, Freer J, Hall JW, Rougier J, Stephenson DB, Wagener T (2016) Sensitivity analysis of environmental models: a systematic review with practical workflow. Environ Model Softw 79:214–232. https://doi.org/10.1016/j.envsoft.2016.02.008
Saltelli A, Ratto M, Andres T, Campolongo F, Cariboni J, Gatelli D, Saisana M, Tarantola S (2008) Global sensitivity analysis. The Primer. Wiley, Chichester
Fenneteau F, Li J, Nekka F (2009) Assessing drug distribution in tissues expressing P-glycoprotein using physiologically based pharmacokinetic modeling: identification of important model parameters through global sensitivity analysis. J Pharmacokinet Pharmacodyn 36:495. https://doi.org/10.1007/s10928-009-9134-8
CDER (FDA) (2016) Physiologically Based Pharmacokinetic analyses—format and content: guidance for industry, draft. Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration (FDA), Rockville
CHMP (EMA) (2016) Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation—draft. Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency (EMA), London
Dressman JB, Fleisher D (1986) Mixing-tank model for predicting dissolution rate control of oral absorption. J Pharm Sci 75:109–116. https://doi.org/10.1002/jps.2600750202
Yu LX, Amidon GL (1999) A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm 186:119–125. https://doi.org/10.1016/S0378-5173(99)00147-7
Saltelli A (2002) Making best use of model evaluations to compute sensitivity indices. Comput Phys Commun 145:280–297. https://doi.org/10.1016/S0010-4655(02)00280-1
Sobol IM (1993) Sensitivity estimates for nonlinear mathematical models. Math Model Comput Exp 1:407–414
Saltelli A, Annoni P (2010) How to avoid a perfunctory sensitivity analysis. Environ Model Softw 25:1508–1517. https://doi.org/10.1016/j.envsoft.2010.04.012
MATLAB R2017b (2017) The MahWorks, Inc., Natick, MA
Archer GEB, Saltelli A, Sobol IM (1997) Sensitivity measures, Anova-like techniques and the use of bootstrap. J Stat Comput Simul 58:99–120. https://doi.org/10.1080/00949659708811825
Lennernäs H, Palm K, Fagerholm U, Artursson P (1996) Comparison between active and passive drug transport in human intestinal epithelial (CACO-2) cells in vitro and human jejunum in vivo. Int J Pharm 127:103–107. https://doi.org/10.1016/0378-5173(95)04204-0
Scherholz ML, Forder J, Androulakis IP (2018) A framework for 2-stage global sensitivity analysis of GastroPlus™ compartmental models. J Pharmacokinet Pharmacodyn 45:309–327. https://doi.org/10.1007/s10928-018-9573-1
McNally K, Cotton R, Loizou GD (2011) A workflow for global sensitivity analysis of PBPK models. Front Pharmacol 2:31. https://doi.org/10.3389/fphar.2011.00031
Daga PR, Bolger MB, Haworth IS, Clark RD, Martin EJ (2018) Physiologically based pharmacokinetic modeling in lead optimization. 2. Rational bioavailability design by global sensitivity analysis to identify properties affecting bioavailability. Mol Pharm 15:831–839. https://doi.org/10.1021/acs.molpharmaceut.7b00973
Simcyp (2017) Simcyp Simulator—Version 17. CERTARA, L. P., Sheffield
Bu H-Z (2006) A literature review of enzyme kinetic parameters for CYP3A4-mediated metabolic reactions of 113 drugs in human liver microsomes: structure–kinetics relationship assessment. Curr Drug Metab 7:231–249. https://doi.org/10.2174/138920006776359329
Sugano K (2008) Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int J Pharm 363:73–77. https://doi.org/10.1016/j.ijpharm.2008.07.002
Manallack DT (2007) The pKa distribution of drugs: application to drug discovery. Perspect Med Chem. https://doi.org/10.1177/1177391x0700100003
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Valetin J (2006) Human alimentary tract model for radiological protection. Ann Int Comm Radiol Prot (ICRP) 36(1–2):25–327
Gastroplus (2017) Gastroplus—Version 9.5. Simulations Plus, Lancaster
Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Parry Howells G, Tipton IH (1975) Report of the task group on reference man. Ann Int Comm Radiol Prot (ICRP) 100:100. https://doi.org/10.1016/0146-6453(79)90123-4
Olivares-Morales A, Ghosh A, Aarons L, Rostami-Hodjegan A (2016) Development of a novel simplified PBPK absorption model to explain the higher relative bioavailability of the OROS® formulation of oxybutynin. AAPS J 18:1532–1549. https://doi.org/10.1208/s12248-016-9965-3
Valetin J (2002) Basic anatomical and physiological data for use in radiological protection: reference values. Int Comm Radiol Prot (ICRP) 32(3):1–277
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484. https://doi.org/10.1177/074823379701300401
Acknowledgements
This work is an in-kind contribution to the OrBiTo Project (http://www.imi.europa.eu/content/orbito).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Melillo, N., Aarons, L., Magni, P. et al. Variance based global sensitivity analysis of physiologically based pharmacokinetic absorption models for BCS I–IV drugs. J Pharmacokinet Pharmacodyn 46, 27–42 (2019). https://doi.org/10.1007/s10928-018-9615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-018-9615-8