Abstract
A series of epoxidized isobutyl esters (EIE) derived from soybean oil deodorizing distillate (SODD) were synthesized via esterification with isobutanol followed by epoxidation. Epoxidized isobutyl soyate (EIS), epoxidized isobutyl soyate distillate (EISD), as well as the epoxidized esters of the main fatty acids contained in SODD, namely, epoxidized isobutyl linoleate (EIL), and epoxidized isobutyl oleate (EIO) were also synthesized and assessed as environmentally friendly plasticizers for polylactide (PLA). A comparison of the plasticizing efficiency of 10 wt.% of these EIE on PLA properties is addressed in this work. The effects of the different EIE on mechanical properties (tensile and impact tests) at 21 ºC, thermal transitions and thermal degradation, dynamic-mechanical thermal properties and dimensional change with temperature, and morphology are evaluated and compared with commercial epoxidized soybean oil (ESBO), and acetyl tributyl citrate (ATBC). Tensile tests indicate that EIE provide increased elongation at break from 8.8% (neat PLA), up to 10–32%, depending on the EIE. EIE seem to be more compatible with PLA as observed by field emission scanning electron microscopy (FESEM) since they do not give evidence of phase separation, or plasticizer saturation, which is clearly observed with ESBO. Regarding thermal properties, all EIE provide a noticeable decrease in the glass transition temperature (Tg) from 61.6 ºC (neat PLA), down to values ranging from 42 to 48 ºC, remarkably lower than the decrease provided by ESBO with a Tg value of 56.6 ºC. These findings reveal that EIE are promising plasticizers for PLA with balanced properties and contribute to improve its intrinsic brittleness by increasing the impact toughness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing social awareness regarding sustainable development issues, as well as concerns related to petroleum depletion act as driving forces for the development of more sustainable formulations of industrial plastics. In recent decades, the growth in the usage and applications of biopolymers has increased exponentially [1, 2]. The group of biopolymers that has attracted the most attention is that comprised by polymers obtained from renewable sources which also offer biodegradability [3, 4]. This group includes polymers derived from polysaccharides (such as cellulose, starch, chitosan, chitin, pectin, and so on) [5,6,7], polymers derived from proteins (such as gluten, soy, zein, casein, among others) [8,9,10,11], and bacterial polyesters such as polyhydroxyalkanoates (PHA) [12,13,14]. Despite the significant advances made in these biopolymers, polylactide (PLA) is by far one of the most industrially used biopolymers. It offers competitive cost, a wide range of commercial grades for injection molding, extrusion, melt spinning, processing conditions like other commodity plastics, and a good balance of properties (mechanical, thermal, barrier, optical, and so on). However, it is a brittle polymer and therefore offers low toughness. Its elongation at break does not exceed 10% in most commercial grades.
It is possible to improve toughness through various strategies, including copolymerization, physical blending, and plasticization [15,16,17,18]. At the industrial level, plasticization is widely used. Plasticizers reduce the glass transition temperature (Tg), thereby increasing the chain mobility due to internal lubrication effect. Numerous plasticizers have been proposed for PLA, both monomeric and polymeric. Among the monomeric plasticizers, citrates (e.g. triethyl citrate-TEC, acetyltributyl citrate-ATBC, tributyl citrate-TBC) stand out [19,20,21], as well as adipates [22], terpenes and terpenoids [23,24,25,26], itaconates [27], and tartrates [28]. Various oligomeric/polymeric plasticizers have also been proposed as efficient plasticizers for PLA, such as oligomers of lactic acid (OLA), [29], polyethylene glycol (PEG) [30], polypropylene glycol (PPG) [31] and polyadipates [32, 33].
The exceptional plasticizing properties of modified vegetable oils for PLA has also been described. Vegetable oils exhibit a triglyceride structure, with a glycerin backbone to which different fatty acids are attached. Unsaturated or polyunsaturated fatty acids such as oleic acid, linoleic acid, and linolenic acid, with 1, 2, and 3 carbon–carbon double bonds, respectively, offer wide possibilities for chemical modification, including hydroxylation, epoxidation, maleinization and acrylation, among others [34,35,36,37]. In the field of plasticization, epoxidation processes are particularly relevant, as they convert unsaturations into oxirane rings through a process of epoxidation with peroxyacids [38]. The use of epoxidized vegetable oils (EVO) is widespread in the polyvinyl chloride (PVC) plasticization sector, with epoxidized soybean oil (ESBO) and epoxidized linseed oil (ELO) standing out as commonly used secondary plasticizers [39,40,41]. Given their particular chemical structure, featuring polar functional groups and hydrophobic chain segments, the use of epoxidized vegetable oils as plasticizers for PLA has been proposed [42, 43]. Garcia-Garcia et al. [44] reported the excellent plasticization efficiency of epoxidized karanja oil (EKO) with a 77.8% increase in elongation at break with 5 wt.% EKO. Carbonell-Verdu et al. [45] also reported good plasticization effect of epoxidized cottonseed oil. They observed an increase in the elongation at break from 9.06% (neat PLA) up to values of 110.5% with 10 wt.% of this epoxidized compound. Bouti et al. [46] also observed this exceptional improvement on ductility by the addition of 20 wt.% epoxidized sunflower oil (ESO) to PLA with an increase in elongation at break from 5% (neat PLA), up to 16%. Additionally, they reported better plasticization effects of epoxidized soybean oil (ESBO) on PLA, compared to epoxidized sunflower oil (ESO). Orue et al. [47] compared the plasticization efficiency of several epoxidized and non-epoxidized vegetable oils. They concluded that epoxidized vegetable oils provide better plasticization to PLA as observed by a remarkable increase in elongation at break and impact strength. On the other hand, epoxidized fatty acid esters (EFAE) are highly compatible additives that can be produced by esterification or transesterification of fatty acids contained in triglycerides with mono- or polyalcohols followed by an epoxidation stage. Utilizing branched and linear alcohols of short to medium chain lengths has been a strategy to enhance plasticity and resist migration [48]. Ferri et al. [49] reported an exceptional increase in the elongation at break of PLA by addition of ≈4.7 wt.% of octyl epoxyoleate, thus showing the potential of individual fatty esters as base materials for PLA plasticization. Liang et al. [50] recently reported the exceptional plasticization of epoxidized fatty acid isobutyl esters as plasticizers for PVC, with a remarkable improvement on extraction resistance and migration.
Soybean oil deodorizer distillate (SODD), a by-product of soybean oil refining, is generated during the deodorization process, which removes compounds that provide color, odor and acidity. It contains 22.7–49.9% of a mixture of fatty acids, and 28.2–65.5% acylglycerides, as well as other bioactive compounds such as tocopherols, squalene, and phytosterols [51,52,53]. SODD represents 0.3–0.5% of the worldwide processed soybean oil and, as it has not a direct application, it is considered an industrial waste [53, 54]. This waste has been proposed as raw material for biodiesel (due to its high percentage of fatty acids and triglycerides), as well as valorization of the abovementioned bioactive compounds [55,56,57,58]. Khatton et al. [52] reported the lipid profile of SODD coming from Indian soybean oil, which consisted on palmitic acid (23.2–25.5%), stearic acid (1.4–2.4%), oleic acid (23.8–26.1%), linoleic acid (40.4–41.1%), and linolenic acid (2.7–3.2%). This makes SODD a valuable by-product for further chemical modification, including esterification and epoxidation, thus widening the potential of these soybean oil derivatives as biobased plasticizers for a wide range of polymers, including PLA. Higher yield on free fatty acids and acylglycerides can be obtained by previous extraction of other valuable compounds. In the case of squalene, conventional distillation is not feasible as it is thermolabile due to the presence of unsaturations. Extraction with supercritical CO2 or Soxhlet extraction followed by silica gel column chromatography, are a very interesting methods to extract and concentrate squalene [58, 59]. With regard to the other valuable compounds, namely tocopherols and phytosterols, they can be extracted and concentrated by a two-step method consisting of a modified Soxhlet extraction followed by a cold (T < 60 ºC) saponification which allows separating free fatty acids and acylglycerols from these valuable compounds [60].
The novelty of this work lies in obtaining epoxidized compounds from an industrial waste from the deodorization of soybean oil, for use in the efficient plasticization of PLA, contributing positively to the transition from linear to circular economies, as well as to achieving various sustainable development goals listed in the 2030 agenda. The primary objective of this investigation is to evaluate the performance of a spectrum of epoxidized isobutyl esters (EIE), derived from soybean oil deodorizing distillate (SODD) as potential biobased plasticizers for polylactide (PLA). Two plasticizers derived from SODD were synthesized, namely epoxidized isobutyl soyate (EIS), and epoxidized isobutyl soyate distillate (EISD). In addition, the main fatty acids contained in SODD were individually esterified and epoxidized to give epoxidized isobutyl oleate (EIO), and epoxidized isobutyl linoleate (EIL), respectively. This work explores the effect of the abovementioned epoxides derived from soybean oil deodorizing distillate (SODD), on their plasticization efficiency on PLA. This thorough assessment encompasses the analysis of mechanical properties at room temperature by tensile and Charpy impact tests, morphology of the fractured surfaces, thermal transitions (glass transition temperature, cold crystallization and melting) and main thermal degradation parameters (onset degradation temperature, maximum degradation rate, and residual mass), as well as dynamic -mechanical thermal properties (storage modulus, loss modulus, and dynamic damping factor with increasing temperature, and the dimensional change and the subsequent coefficient of linear thermal expansion. Moreover, an assessment of the polymer-plasticizer chemical interactions of the proposed plasticizers incorporated into PLA injection-molded parts. Acetyl tributyl citrate (ATBC), and epoxidized soybean oil (ESBO) were chosen as reference plasticizers for comparative evaluation of performance.
Experimental
Materials and Reagents
Soybean oil deodorizing distillate (SODD) was kindly supplied by Team Foods (Bogotá, Colombia). The lipid profile of the supplied SODD was: C16:0 (13.79%), C18:0 (4.91%), C18:1 (30.00%); C18:2 (47.61%), C18:3 (3.70%). Table 1 shows a summary of the main physicochemical properties of SODD. The acid value was determined according to ISO 660 by the filtration method. With regard to the iodine value, it was determined according to ISO 3961. The peroxide value was obtained by following the guidelines of ISO 3960. The saponification number was determined according to ISO 3657, while unsaponifiable matter was measured according to ISO 3595.
Food grade linoleic acid, and technical grade oleic acid were supplied by Sigma Aldrich, and QuimiEsencias (Bogotá, Colombia), respectively. Esterification of SODD and fatty acids was carried out by reacting with isobutanol (> 99% GC, Merck) using sulfuric acid (96% wt., Merk) as a catalyst. Glacial acetic acid (Merk), aqueous hydrogen peroxide (50 wt.%, Chemi), and Amberlite IRC 120 H (Merck) were used to generate the peroxyacids in situ to carry out the epoxidation reactions of the synthesized esters. Absolute ethanol USP (PanReac), toluene (99.98% wt., J.T. Baker), sodium hydroxide (99% wt., Supelco), chloroform (HPLC grade, J.T. Baker), sodium thiosulphate pentahydrate (Supelco), Wijs reagent (0.2N, PanReac), hydrogen chloride (0.5 M Panreac), and 2-propanol USP (PanReac) were used for the determination of the effectiveness of the epoxidation process by measuring the acid and iodine values, as well as the oxirane oxygen content.
Synthesis of Isobutyl Esters (IE) from SODD
Isobutanol-derived esters from fatty acids and SODD, specifically isobutyl oleate (IBO), isobutyl linoleate (IBL), and isobutyl soyate (ISO), were synthesized via Fisher esterification of oleic acid, linoleic acid, and SODD with isobutanol. This process employs a 5:1 molar ratio of fatty acids to isobutanol, with 2 wt.% sulfuric acid as a catalyst. The reaction was conducted in a 150 mL four-necked glass reactor with a magnetic stirrer, and a thermostatic bath (MV-4, Julabo) to keep temperature constant at 94 °C under inert N2 atmosphere. Additional components included a Vigreux column, condenser, and cylindrical separatory funnel for condensate recovery. The reaction time was set to 6 h, and after that time neutralization was accomplished with an alcoholic saturated sodium hydroxide solution in isobutanol. This was followed by a vacuum distillation stage. Subsequent neutralization was performed until the acid value decreased below 0.06 mg KOH/g. The resulting solution underwent washing until achieving a neutral pH, followed by vacuum drying of the synthesized esters. Additionally, the isobutyl ester of SODD was subsequently distilled at 180 ºC and 0.1 mbar to obtain an isobutyl ester of oleic acid-rich fraction and was further subjected to epoxidation as the other esters.
Epoxidized Isobutyl Esters (EIE) from SODD
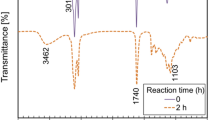
Unsaturations contained in the different isobutyl esters were subjected to epoxidation with peracetic acid generated in situ to obtain the corresponding epoxidized esters, namely epoxidized isobutyl oleate (EIO), epoxidized isobutyl linoleate (EIL), epoxidized isobutyl ester of SODD or soyate (EIS), and epoxidized isobutyl ester of SODD distillate (EISD). To carry out the epoxidation, the unsaturation to hydrogen peroxide molar ratio was set to 1:1.5. A 2 wt.% Amberlite IRC 120 H strongly acidic cation exchange resin was used as catalyst. Epoxidation was carried out in a 150 mL five-necked glass reactor at a temperature of 60 ºC. A thermometer was connected to control the reaction temperature in addition to two thermostatic baths with heating and cooling fluids, respectively. The setup included a calibrated syringe pump to appropriately dose hydrogen peroxide and a reflux condenser to recover volatiles. After completion of the epoxidation reaction at 3 h, the resulting product was placed into a glass funnel. The water phase was discarded, while the oily phase was neutralized with a 5 wt.% bicarbonate solution. The resulting epoxides were rinsed with distilled water at 40 ºC until neutral pH was obtained in the rinsing water. Finally, to remove the residual moisture, the corresponding epoxides were dried over a magnesium sulphate salt. Same methods described in Sect. "Materials and reagents." were followed to fully characterize the obtained epoxides. Additionally, the oxirane oxygen content, was measured by following the guidelines of the NTC 2366 standard. The moisture content was obtained using the Karl Fisher volumetric titration method. Scheme 1 gathers the chemical structure of SODD, the mina reactions, and the chemical structure of the epoxidized isobutyl esters of SODD. The optimum epoxidation conditions were obtained for isobutyl linoleate by calculating the double bond conversion (DBC), and the yield to oxirane oxygen as indicated in Eqs. 1 and 2
Where II0 is the initial iodine value of the sample, and II is the changing iodine value along the reaction (taken at the end of the reaction time, for the overall conversion).
where OOexp refers to the obtained experimental oxirane oxygen (%), while OOmax stands for the theoretical oxirane oxygen (%) which is 8.4% for isobutyl linoleate used as model compound for the epoxidation optimization.
Processing of Plasticized PLA Formulations with Epoxidized SODD Derivatives
As PLA is very sensitive to hydrolysis, PLA pellets underwent drying within an air circulating oven at 80 ºC for a 12 h to eliminate moisture. Subsequently, PLA was mixed with 10 wt.% of the different synthesized epoxidized isobutyl esters from SODD. The formulations were compounded in a 15-cc twin-screw mini-extruder Xplore MC 15 HT from Xplore Instruments BV (Sittard, The Netherlands). To allow plasticization, all mixtures were homogenized at 190 ºC for 3 min at a rotational speed of 100 rpm. Subsequently, the plasticized formulations were charged into an extractable chamber of a 12-cc micro-injection molding machine Xplore IM 12 from Xplore Instruments BV (Sittard, The Netherlands), and injected at 190 ºC into standard samples for further characterization. To prevent PLA samples from hydrolysis, all specimens were stored in a vacuum desiccator at room temperature.
Spectroscopic Characterization
Chemical interactions within the PLA and plasticizers were investigated through attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR). Spectra were acquired using a Bruker S.A Vector 22 spectrometer (Madrid, Spain) with a PIKE MIRacleTM single reflection diamond ATR accessory (Madison, Wisconsin, USA). Data were gathered as the average of 20 scans spanning from 4000 to 500 cm⁻1, with a spectral resolution of 2 cm⁻1.
Thermal Transitions and Thermal Degradation Characterization
The thermal characteristics of both PLA and plasticized PLA formulations incorporating epoxidized isobutyl esters from SODD were assessed through differential scanning calorimetry (DSC) utilizing a DSC-821 calorimeter from Mettler-Toledo Inc. (Schwerzenbach, Switzerland) under a N2 atmosphere (50 mL/min). Samples weighing between 5 to 10 mg were subjected to an initial heating stage from 25 to 180 ºC to eliminate any prior thermal history. Subsequently, a cooling stage to 0 ºC was scheduled to cool down under controlled conditions. Lastly, a second heating stage up to 300 ºC was implemented to obtain the main thermal parameters. The heating/cooling rate was 10 ºC/min for all three stages. These parameters included the glass transition temperature (Tg), the cold crystallization peak temperature (Tcc), the melt peak temperature (Tm), as well as the enthalpies corresponding to the cold crystallization (ΔHcc), and melting (ΔHm) processes, which were derived from the second heating cycle.
Thermal degradation analyses were conducted on both PLA and its plasticized formulations with epoxidized isobutyl esters of SODD derivatives using thermogravimetric analysis (TGA) with a TG-DSC2 thermobalance from Mettler Toledo (Columbus, Ohio, USA). Samples weighing between 7 to 10 mg were placed in standard alumina cylindrical crucibles of 70 μL capacity and sealed with lids. Subsequently, these samples underwent dynamic heating from 30 ºC to 700 ºC under a nitrogen atmosphere (flow rate: 50 mL/min) at a heating rate of 20 ºC/min. Key thermal parameters extracted from TGA included the onset degradation temperature (T5), defined as the temperature at which a 5% mass loss occurred, and the maximum degradation rate temperature (Tmax), corresponding to the peak maximum temperature of the first derivative DTG curve. All samples underwent triplicate analyses via DSC and TGA to ensure the reliability of the results.
Mechanical Properties
Tensile properties of injection-molded samples of plasticized PLA formulations containing epoxidized isobutyl esters from SODD were evaluated using an ELIB 50 universal testing machine from S.A.E. Ibertest (Madrid, Spain) according to ISO 527–3:2018 equipped with a load cell of 5 kN. Dog-bone samples were clamped and subjected to a crosshead speed of 10 mm/min. At least five specimens of each formulation were tested, and the main tensile properties, namely the maximum tensile strength (σmax), the elastic modulus (E), and the elongation at break (%εb) were measured and averaged.
The impact energy absorption of the specimens was assessed in accordance with ISO 179:2010 utilizing a Charpy test apparatus equipped with a 6-J pendulum sourced from Metrotec S.A. (San Sebastián, Spain). The absorbed energy for each plasticized PLA formulation was computed based on the average energy absorbed by five distinct unnotched specimens.
Morphology
The morphology of the fractured samples from impact tests of neat PLA and plasticized PLA formulations with epoxidized isobutyl esters of SODD derivatives, was examined by field emission scanning electron microscopy (FESEM). Initially, the samples underwent sputter coating with a gold–palladium alloy using an EMITECH sputter coating mod. SC7620 from Quorum Technologies, Ltd. (East Sussex, UK). Subsequently, FESEM images were captured using a ZEISS ULTRA 55 microscope from Oxford Instruments (Abingdon, United Kingdom), operating at 2 kV.
Dynamic-mechanical Thermal Properties and Dimensional Stability with Temperature
Dynamic mechanical thermal analysis (DMTA) was conducted using a Mettler Toledo DMA1 instrument (Columbus, Ohio, USA) on samples working in single cantilever. The analysis was performed at a frequency of 1 Hz, with a maximum amplitude of 10 μm in the free cantilever. Samples measuring 25 × 10 × 4 mm3 were subjected to a temperature sweep from -50 ºC to 100 ºC, at 2 ºC/min. The effect of increasing temperature on the storage modulus (E’), the loss modulus (E”), and the dynamic damping factor (tan δ) was studied.
To assess the dimensional stability of both neat PLA and plasticized formulations containing epoxidized isobutyl esters of SODD derivatives, thermomechanical analysis (TMA) was employed. A TMA instrument, model Q400 from TA Instruments (New Castle, Delaware, USA), was utilized for this purpose. Samples measuring 10 × 10 × 4 mm3 underwent heating from -50 ºC to 120 ºC at 2 ºC/min, with an applied force of 20 mN. The coefficient of linear thermal expansion (CLTE) was determined by calculating the slope in the linear regions below the onset of the glass transition, and above the endset of the cold crystallization process. All DMTA, and TMA tests were conducted in triplicate to ensure the reliability of the obtained results.
Results and Discussion
Characterization of the Epoxidized Isobutyl Esters from SODD
Table 2 summarizes the main physicochemical properties of the obtained epoxides. The double bond conversion, DBD(%) obtained for the epoxidation conditions was 89% while the oxirane oxygen yield was comprised in the 62%-75%. This suggests that moderate oxirane cleavage is achieved at the mild epoxidation conditions used in this research. The highest oxirane oxygen % is obtained for EIL with a value of 6.3%, which is consistent with the values reported in literature due to the high unsaturation content of linoleic acid which could lead to such high values up to 8% [61, 62]. With regard to EIS and its distillate EISD, the oxirane oxygen % is around 4.1%, which is slightly lower to epoxidized soybean oil (ESBO) [63], with approx. 4.3 epoxy group per triglyceride. The obtained oxirane oxygen % for EIS and EISD is similar to that reported to high oleic acid soybean oil, with a value of around 4.4% [62]. Anyway, the exceptional plasticization properties of epoxidized vegetable oils on PLA, has been widely reported in literature, covering a wide range of oxirane oxygen of 8.3% (epoxidized linseed oil) [64], 5.5% (epoxidized sunflower oil) [46],3.5% (epoxidized karanja oil) [65], 4.22% (Brazil nut oil) [66], 2.84% (epoxidized palm oil) [67]. Therefore, as the oxirane oxygen content of the SODD derivatives is comprised between 4.1 – 4.2%, it is expected these compounds can provide good plasticization properties to PLA.
On the other hand, there are different ways to assess the plasticization efficiency, depending on the primary expected effect. In the case of PLA, the plasticization efficiency is a combination of mechanical properties and thermal transitions. With regard to the thermal transitions, PLA shows a glass transition (Tg) of 55 – 65 º C, and the cold crystallization peak, (Tcc) is located at 98 – 105 ºC. With regard to mechanical properties, the tensile strength of PLA changes in the 50 – 70 MPa range, while the elongation at break is comprised between 3 – 10%. Finally, the impact strength of unnotched PLA is 32 kJ/m2. Therefore, the plasticization efficiency can be assessed by measuring the decrease in Tg and Tcc, as well as by a decrease in tensile strength and an increase in ductile properties (elongation at break and impact strength). These criteria are discussed in the following sections.
Chemical Interactions in Plasticized PLA with Epoxidized Isobutyl Esters of SODD Derivatives
FTIR spectroscopy was employed to observe the shifting of absorption peaks within designated spectral regions, facilitating the identification of specific functional group interactions between PLA and the selected epoxidized compounds from SODD used as plasticizers. Figure 1a gathers the FTIR spectra of the individual plasticizers used in this study, while Fig. 1b shows the FTIR of plasticized PLA formulations containing 10 wt.% each plasticizer. As it can be seen in Fig. 1a, acetyltributyl citrate (ATBC) shows the typical peaks located at 1736 cm−1, and 1178 cm−1, assigned to the stretching vibrations of ester carbonyl groups, and stretching vibration of the –C–O– group of the ester, respectively [68]. Regarding all the epoxidized compounds, in addition to the abovementioned peaks corresponding to the ester group, their position is slightly shifted to 1734 cm−1 (–C = O), and 1170 cm−1 (–C–O–), respectively. In addition, the oxirane ring shows a typical peak at 822 cm−1 (usually in the 860 – 790 cm−1 band) [69,70,71], and a peak at 1242 cm−1 attributed to the C–O–C asymmetric stretching of the oxirane ring [72]. Epoxidized compounds also show other peaks related to the hydrocarbon chain: 724 cm−1 attributed to (CH2)n rocking vibration, two peaks at 1466 cm−1, and 1380 cm−1, which correspond to the C–H bending (scissoring in CH2), and C–H symmetric bending in CH3. Other characteristic peaks can be seen at 1748 cm−1, which is attributed to the –C = O group, while two strong peaks at 1080, and 1182 cm−1 correspond to the –C–O– asymmetric and symmetric vibrations respectively. Moreover, the peak located at 868 cm−1 corresponds to the amorphous PLA phase, while the peak centered at 756 cm−1 is related to the crystalline PLA regions [73]. Regarding the plasticized PLA formulations containing different epoxidized isobutyl esters of SODD, Fig. 1b shows the comparative spectra. Neat PLA shows characteristic peaks at 2920 cm−1, and 2852 cm−1, ascribed to the –CH stretching of –CH < and – CH3. Moreover, it shows a peak at 1456 cm−1 assigned to the scissoring bending of –CH group in PLA chains. The peak located at 1364 cm−1 corresponds to the –CH symmetric bending of methyl groups, while characteristic – CH rocking vibration can be detected by the peak at 868 cm−1 [72].
Regarding to the spectra of PLA with the different SODD-derived plasticizers, it is worthy to note that there is high similarity between the most relevant peaks and bands corresponding to individual PLA and all SODD-derivatives, but it is important to remark that the peaks located at 822 cm−1, and 1242 cm−1, characteristics of the epoxy ring, have disappeared. This could be related to a dilution effect since the spectra of the plasticized PLA with epoxidized SODD-derivatives are a linear combination of weighed individual spectra. Despite this, some authors have reported the disappearance of these characteristic peaks in plasticized PLA with epoxidized vegetable oils or derivatives, that may be attributed to some interaction between the epoxy rings and hydroxyl terminal groups in PLA chains by hydrogen bonding [72, 74], which could support the good plasticization efficiency of these compounds.
Thermal Characterization of Plasticized PLA with Epoxidized Isobutyl Esters of SODD Derivatives
The plasticization efficiency is closely linked to the reduction of the glass transition temperature (Tg). The incorporation of plasticizers leads to an increase in the free volume, reducing the intensity of polymer-to-polymer interactions as widely reported in literature [75, 76]. This, in turn, enhances the mobility between polymer chains, consequently reducing Tg and improving the ductile properties of the material. PLA is a polymer with a relatively high Tg, around 61.6 ºC, as shown in Fig. 2a, corresponding to the glass transition temperature range from DSC characterization. Consequently, at room temperature, PLA is below its Tg, resulting in a glassy state polymer. With the incorporation of 10 wt.% ATBC, the Tg decreases to values of 46.3 ºC, demonstrating the efficiency of ATBC on PLA plasticization, and in accordance to the previous plasticization efficiency criteria. With the incorporation of 10 wt.% of the epoxidized isobutyl esters of oleic acid (EIO) and linoleic acid (EIL), Tg decreases to similar values as those observed with ATBC, even to lower values (42 – 43 ºC), providing a clear plasticization effect. EIL provides the lowest Tg, which could be related to its higher oxirane oxygen content of 6.31%, since it has more oxirane groups per molecule (see Scheme 1), compared to all other epoxidized isobutyl esters, with an oxirane oxygen content around 4.0 – 4.5%, (see Scheme 1) as reported by Dominguez-Candela et al. [77], in plasticized PLA with epoxidized chia seed oil, with an average oxirane oxygen content of 6.7%. Among all the epoxidized plasticizers used in this work, the one offering the least reduction in Tg is epoxidized soybean oil (ESBO), with a Tg value of 56.6 ºC. It is important to bear in mind that ESBO has a triglyceride (it is a triacylgrlyceride) branched structure with a molecular weight above 950 g/mol, which restricts chain mobility due to its particle miscibility with the PLA matrix. These results are consistent with the values reported by Ali et al. [78], who observed a decrease in Tg from 60.4 ºC down to 54.3 ºC for plasticized formulation containing 10 wt.% ESBO, and Sempere-Torregrosa et al. [79], who observed a similar trend in plasticized PLA with epoxidized corn oil (ECO). The epoxidized isobutyl esters of SODD offer an intermediate plasticization efficiency, with Tg values for the undistilled soyate (EIS) of 48.3 ºC, and a lower value of 44.6 ºC for the distilled soyate (EISD). These results demonstrate the exceptional plasticization that SODD derivatives can provide to PLA with Tg values lower than other epoxidized fatty acid esters as reported by Ferri et al. [49], who observed a decrease in Tg from 64.2 ºC (neat PLA) down to 57 ºC for the plasticized PLA formulation containing 10 wt.% octyl epoxyoleate, thus suggesting the isobutyl pendant group positively contributes to increase the free volume and hence, improving the plasticization efficiency, with comparable results to those obtained with epoxidized soyate methyl esters [80].
DSC thermograms corresponding to different thermal transitions of neat PLA and plasticized PLA formulations with epoxidized isobutyl esters of SODD, obtained from the 2nd heating cycle: (a) glass transition temperature range, (b) cold crystallization process, and (c) pre-crystallization and melt peak process
Another interesting effect linked to the increase in the free volume and polymer chain mobility is the shift of the cold crystallization process towards lower temperatures, as observed by Maiza et al. [81], in plasticized PLA formulations containing different citrates, namely TEC and ATBC. As observed, the cold crystallization peak of PLA is wider and shorter compared to those corresponding to the plasticized formulations. This suggests a broader distribution of crystals in neat PLA with a cold crystallization maximum temperature peak, Tcc, of 98.3 ºC, which is reduced in a similar trend as observed for Tg values. The lowest Tcc is obtained for the plasticized formulation with 10 wt.% EIO, with values around 80.4 ºC (Fig. 2b). Epoxidized isobutyl esters of SODD contain hydrophobic moieties (-CH2-), and polar moieties (ester and oxirane groups) (see Scheme 1). This dual functionality exerts a positive effect on polymer chain mobility, as these plasticizer molecules are placed between polymer chains and provide a lubricity effect. On the other hand, the polar groups in plasticizer molecules, can establish interactions with the polar groups in PLA polymer chains thus avoiding exudation of the plasticizer. It has been proposed that epoxidized esters may act as efficient crystallization agents due to the increased chain mobility these plasticizers provide, which, in turn, has a positive effect on crystallization at lower temperatures. As previously stated, plasticizers increase the free volume, reducing the intensity of the polymer-to-polymer interactions; hence, the chain mobility is triggered at lower temperatures [82], resulting in a subsequent increase in the crystallinity developed during the cold crystallization, as can be seen by a general increase in the cold crystallization enthalpy, ΔHcc values, as shown in Table 3.
It is interesting to note the presence of a small exothermic crystallization peak (Fig. 2c), just before the melting process [83], which is associated with additional crystallization. Zhang et al. [84], have attributed this exothermic peak to a disorder-to-order phase transition in PLA crystals. Consistent with the cold crystallization process, the peak temperature of this additional crystallization also shifts to lower temperatures with the incorporation of plasticizers. It decreases from 156.9 ºC for neat PLA to as low as 146.0 ºC for plasticized PLA with 10 wt.% EIO, which exhibited the lowest Tg and Tcc values as observed in Table 3.
Regarding the melting process (Fig. 2c), plasticizers induce a slight reduction in the peak melting temperature, Tm. Neat PLA exhibits a Tm of 173.5 ºC, and a slight decrease to values around 170 ºC is observed for almost all plasticized formulations with 10 wt.% ATBC and the epoxidized isobutyl esters of SODD (Table 3). Maiza et al. [81] have demonstrated a similar decrease in the Tm of neat PLA, from 152.03 ºC down to values of 150.08 ºC and 147.97 ºC with 10 wt.% ATBC and 10 wt.% TEC, respectively. Regarding the maximum crystallinity that PLA can develop, the melting enthalpy values, ΔHm, are similar for all samples, in the range of 49–55 J/g, except for the PLA formulations plasticized with 10 wt.% EIL and EIO, where it slightly decreases to values around 43 J/g, indicating slightly lower maximum crystallinity values.
In relation to high-temperature thermal stability, thermogravimetric characterization has been carried out (see Fig. 3). Epoxidized vegetable oils (EVOs) and their corresponding esters have been widely used in PVC plasticization as secondary plasticizers, as they enhance thermal stability against degradation by blocking the HCl released during degradation [85,86,87]. Epoxidized vegetable oils and their esters have also proven to provide extra thermal stability to PLA. Al-Mulla et al. [88] confirmed a noticeable increase in the onset degradation temperature of PLA from 236.41 ºC up to 283.32 ºC by the addition of 5 – 30 wt.% epoxidized palm oil (EPO) as a plasticizer. Quiles-Carrillo et al. [89] also showed the exceptional thermal stabilization of acrylated epoxidized soybean oil (AESO) (5.0 – 7.5 wt.%) on PLA. Bouti et al. [46] observed this phenomenon in plasticized PLA with epoxidized sunflower oil with different oxirane content. Ferri et al. [49] also confirmed slight thermal stabilization phenomenon on plasticized PLA with different amounts of an epoxidized fatty acid ester, namely octyl epoxyoleate. They observed very slight thermal stabilization at the onset degradation, while this effect was more pronounced at the maximum degradation rate temperature (Tmax). Despite this, Sustaita-Rodríguez et al. [90] have obtained similar results to those observed in this study. In particular, they observed a decrease in the onset degradation temperature (T5), from 325.7 ºC (neat PLA) to values lower than 300 ºC for all formulations containing different amounts of epoxidized fatty acid esters from grape seed. As it can be seen in Fig. 3a, the TGA curve of PLA shows the typical degradation profile of a polymer by chain scission in a single step. As observed in the inlet zoom image in Fig. 3a, the TGA curves of the plasticized PLA formulations with ESBO, and EIS are shifted to higher temperatures, thus giving evidence of an improvement in thermal stability. Regarding the onset degradation temperature, measured at 5% mass loss (T5), neat PLA presents a T5 of 338.4 ºC. This is reduced to 309.5 ºC for ATBC-plasticized PLA formulation which is in accordance with the results reported by Maiza et al. [19], who observed a noticeable decrease in the onset degradation temperature as the citrate-type plasticizer (TEC or ATBC) content increased in plasticized PLA formulations with up to 30 wt.% both citrates. The T5 for the ESBO-plasticized PLA is higher than neat PLA, with values around 343.3 ºC, thus corroborating the abovementioned thermal stabilization effect of high molecular weight epoxidized vegetable oils. Nevertheless, all the epoxidized isobutyl esters of SODD show a slight decrease in the T5 value, close to 320 ºC, except that of epoxidized isobutyl soyate (EIS) with a T5 value of 332.8 ºC, which is quite close to that of neat PLA. This could be attributed to the presence of natural antioxidants contained in SODD, such as tocopherols [56, 91], since the T5 for the distillate epoxidized isobutyl soyate (EISD), shows a decrease in T5 down to 322.1 due to the distillation process that can remove these natural antioxidants. Similar tendency, but less pronounced, was observed for the maximum degradation rate temperature, Tmax, obtained from DTG curves (Fig. 3b), which changed from 375.4 ºC for neat PLA down to values of 370 – 375 ºC for ATBC and all other epoxidized isobutyl esters except ESBO which gave an increase in Tmax up to 380.4 ºC, which was expected. The residual mass for all materials was less than 1%. Anyway, the thermal stability of plasticized PLA with epoxidized isobutyl soyates (EIS, and EISD) is not compromised as both T5 and Tmax values suggest (Table 4).
Mechanical Properties of Plasticized PLA with Epoxidized Isobutyl Esters of SODD Derivatives
In Table 5, the effect of different plasticizers derived from SODD on the mechanical properties of PLA is shown. The neat PLA exhibits a tensile strength of 69.9 MPa, along with a modulus of 831 MPa and a relatively low elongation at break of 8.8%. The incorporation of plasticizers into PLA results in a reduction of the mechanical resistance properties (tensile modulus, and tensile strength) due to the increase in free volume between polymer chains which in turn, decreases the polymer chain-to-polymer chain interactions. Tian et al. [92], reported this phenomenon by the addition of 15–20 wt.% ATBC to PLA, and proposed a mechanism of increasing tensile strength by incorporating small amounts of nanofibrillated cellulose to plasticized formulations. Conversely, ductile properties (elongation at break) are increased due to an enhanced polymer chain mobility, with a subsequent decrease in the glass transition temperature (Tg), resulting in improved toughness [80]. In materials plasticized with ATBC, the reduction in mechanical strength properties is evident, with tensile strength and tensile modulus values of 57.6 MPa and 768 MPa, respectively. However, a clear increase in elongation at break is not observed, which remains at values of 8.3%. Overall, to achieve a noticeable plasticizing effect on ductile properties, a plasticizer threshold comprised between 10 – 20 wt.% or higher is required, despite the reduction in Tg observed in the thermal characterization [93]. Baiardo et al. [94] confirmed this phenomenon. They concluded that depending on the chemical structure of the plasticizer, as well as its molecular weight and polarity, among others, this threshold changes. They observed a noticeable increase in elongation at break of neat PLA (1.6%) by plasticizing it with 10 wt.% polyethylene glycol (PEG – 400 g/mol) up to 140%. Nevertheless, this threshold was shifted to higher values (20 wt.%) by incorporating a high molecular weight PEG (10,000 g/mol), which possessed very low elongation at break values below this threshold. With regard to ATBC-plasticized PLA, they observed that the threshold was 12.5 wt.%. With this ATBC content, the elongation at break improved up to 218%, while lower ATBC content gave quite lower elongation at break values (below 7%). Bouti et al. [46] have reported this threshold for plasticized PLA with epoxidized sunflower oil (ESO). They observed a very slight increase in elongation at break for an ESO content of 10 wt.%, while optimum ductile properties were obtained for an ESO content of 20 wt.%, with a noticeable decrease in its mechanical performance.
Both epoxidized isobutyl esters of the main fatty acids in SODD, namely EIL and EIO, show a clear plasticizing effect, even at 10 wt.%, with a tensile strength around 49–50 MPa, and a tensile modulus slightly lower than that of neat PLA. The increase in elongation at break up to values between 12.0 and 13.0% is noteworthy, representing a percentage increase of 36.4%−47.7%, respectively, compared to neat PLA. Epoxidized soybean oil (ESBO) provides excellent ductile properties to PLA at this concentration, with an elongation at break of 32% (representing a percentage increase of 263.6% compared to neat PLA), consistent with the results reported by Bouti et al. [46], who observed an increase in elongation at break in PLA/ESBO blends from around 5% (neat PLA) to 19% with 10 wt.% ESBO. Although the decrease in the Tg of PLA plasticized with 10 wt.% ESBO is not as significant as with the other epoxidized plasticizers derived from SODD and ATBC, the net effect on the increase in elongation at break is attributed to the localized plasticization of low molecular weight ESBO microdroplets finely dispersed in the PLA matrix, as suggested by Piorkowska et al. [95]. With regard to the epoxidized isobutyl soyates (EIS, and EISD), the elongation at break improves to values of 10% for both plasticizers, which represents a percentage increase of 13.6% compared to neat PLA. The tensile strength, as expected, is lower than that of neat PLA, reaching values of 47.5–50.0 MPa, which agree with the results reported by Sustaita-Rodríguez et al. [90] in plasticized PLA with epoxidized methyl esters of a mixture of fatty acids contained in grape seed oil. Regarding the toughness, neat PLA has an impact strength of 32 kJ/m2, and it is not remarkably improved by using 10 wt.% ATBC, epoxidized isobutyl soyates (EIS, and EISD), and epoxidized soybean oil (ESBO), with values around 30 – 34 kJ/m2. Nevertheless, it is worthy to remark the improvement in toughness provided by the epoxidized isobuyl esters of oleic acid (EIO) and linoleic acid (EIL), with impact strength values of 40 kJ/m2 (25% higher to PLA), and 50 kJ/m2 (56.3% higher than neat PLA), for the plasticized PLA formulation with 10 wt.% EIO, and EIL, respectively. Similar results were reported by Ferri et al. [49] in plasticized PLA with epoxidized octyl oleate. They observed an increase in the impact strength (Charpy) from 30.9 kJ/m2, up to values of 40 kJ/m2 for plasticizer content in the 1–17 wt.% range, except for a content of 4.7 wt.% which gave the maximum impact strength value (54.2 kJ/m2). Anyway, the increase in impact strength provided by the plasticizer was superior to neat PLA. Tee et al. [96] reported that PLA plasticized with ESBO gave a slight increase in impact toughness at low concentrations (< 10 wt.%), which agrees with the herein obtained results. Zych et al. [80] have observed an exceptional increase in the toughness of PLA plasticized with epoxidized soybean oil methyl esters (ESOME), by measuring the area under the tensile stress–strain curves. They reported the maximum toughness (76.6 MJ/m3) for the plasticized PLA with 10 wt.% ESOME, which was notably higher than that of neat PLA (0.4 MJ/m3).
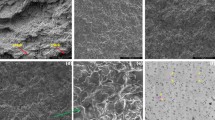
Figures 4 and 5 show the FESEM images of the fractured surfaces of neat PLA and its plasticized formulations with the epoxidized isobutyl esters derived from SODD, taken at different magnifications. At a low magnification of 500 × (Fig. 4), the fracture surface of neat PLA sample (Fig. 4a) shows a typical flat and smooth surface with no evidence of plastic deformation, which corresponds to a brittle polymer. This flat surface is also observable in Fig. 5a which suggests the absence of plastic deformation of a brittle material, with a sharp angle fracture cracks. Regarding the plasticized sample with ATBC (Figs. 4b and 5b), the fracture surface morphology changes in a remarkable way, with a rougher surface exhibiting a wave-type fracture, which is representative for plastic deformation. The zoomed image (Fig. 5b) shows clear evidence of plastic deformation, with filaments and rounded fracture planes resulting from plastic deformation. Regarding the plasticized PLA with epoxidized isobutyl esters of fatty acids (EIO, and EIL), a similar fracture surface with a wave-like morphology related to numerous microcracks formation and growth are clearly identified at low magnification (Figs. 4c and 4d). These morphologies are in total accordance with the previous mechanical properties, which showed the highest impact strength for these two epoxidized compounds. At higher magnification (Figs. 5c and 5d), the plastic deformation is more evident with the presence of rounded fracture planes, and the presence of many filaments attributable to plastic deformation. These morphologies have been described by Ferri et al. [49] in plasticized PLA with an epoxidized oleic acid octyl ester. It is important to note that no signal of phase separation can be detected for plasticized PLA with EIO, and EIL while this phenomenon is observed in plasticized PLA with ESBO (Fig. 5e), with the presence of numerous empty microvoids which suggests an excess plasticizer resulting in this typical morphology as reported by Ali et al. [78] in plasticized PLA with ESBO. This could be related to the higher miscibility of low molecular weight epoxidized isobutyl esters compared to the higher molecular weight of epoxidized soybean oil with triglyceride structure. Regarding the epoxidized isobutyl esters of SODD, their fracture surface is different to the previously wavy surfaces resulting from plastic deformation as can be seen in Figs. 5f and 5g. In fact, no clear evidence of plastic deformation is observed despite rougher surfaces are obtained for EISD. In the case of EIS-plasticized PLA, the fracture morphology is more similar to the brittle neat PLA, with sharp angle fracture lines, and quite flat surfaces, which is consistent with the previous mechanical properties of these formulations.
Thermomechanical Properties of Plasticized PLA with Epoxidized Compounds
Figure 6 shows the dynamic mechanical properties of the neat PLA and its plasticized formulations with epoxidized isobutyl esters of SODD. As it can be observed in Fig. 6a, the evolution of the storage modulus, E’ with increasing temperature shows the main thermal transitions of PLA. Below 50 ºC, the E' practically does not vary with temperature, as the material is in a glassy state. As the temperature increases in the 55º C–75 ºC, a remarkable decrease in E’ (threefold) can be observed, indicating a dramatic softening of the material. This temperature range corresponds to the glass transition temperature, Tg. Another phenomenon can be observed in the temperature range comprised between 75 ºC–90 ºC. As it can be seen in Fig. 6a, the storage modulus, E’, increases in this temperature range by one order of magnitude above the glass transition temperature range, Tg. This is related to the cold crystallization process since the packed crystal structure leads to increased mechanical resistance. In general, the storage modulus, E’ curves for plasticized PLA formulations are shifted to lower temperatures [80], thus confirming the softening of the plasticized materials. This behaviour also confirms the previous results observed by DSC, since both the glass transition temperature (Tg), and the cold crystallization process are shifted to lower temperatures [49]. It is possible to use several criteria to indicate the Tg, by the onset decrease of E’, and the peak maximum of E” (Fig. 6b), or tan δ (Fig. 6c). Different criteria lead to slightly different Tg values, since actually, Tg is not a unique temperature, it involves a temperature range in which the change from a glassy state to a rubber-like state occurs. As can be seen in Table 6, the results obtained by DMTA are consistent with those shown previously by DSC. The Tg values obtained by using the peak maximum of E” are almost coincident with those obtained by DSC, with EIL (epoxidized isobutyl ester of linoleic acid), being the plasticizer which provides the highest decrease in Tg. On the other hand, DMTA results also showed that the ESBO as the plasticizer exhibited the lowest decrease in Tg. Epoxidized isobutyl soyates (EIS, and EISD), show an in-between behaviour. The curves of loss modulus, E”, also give interesting information about both the glass transition (peak maximum), and the cold crystallization (increase in E” at high temperature). Ljungberg et al. [97], reported this similar behaviour regarding the loss modulus in plasticized PLA with 20% triacetine. They observed a remarkable change in the loss modulus curve between a fully crystallized PLA (50 ºC for 3 days), and the immediately processed plasticized PLA. Therefore, the evolution of the loss modulus is also a good tool to follow the cold crystallization process, as well as the effectiveness of the plasticization, since below Tg, all loss modulus curves of plasticized PLA are shifted to high values as reported by Ljungberg et al. [98], in plasticized PLA with citrate and malonate plasticizers.
Thermomechanical analysis (TMA) yields insightful findings regarding the influence of the different epoxidized isobutyl esters from SODD on PLA. Figure 7 illustrates the dimensional change in plasticized PLA compositions with these plasticizers, while key TMA findings are summarized in Table 7. TMA allows the assess the relationship between PLA dimensional variation and the thermal processes (glass transition and cold crystallization). Below its glass transition temperature (Tg), notably under 50 ºC, a linear correlation exists between temperature rise and dimensional change. The glass transition process correlates with material softening, consequently, leading to expansion in response to rising temperatures. The onset of the glass transition temperature can be assessed as a shift in the linear slope within temperatures below Tg (black arrows in Fig. 7).
At temperatures above the glass transition temperature (Tg), PLA undergoes cold crystallization, resulting in a denser molecular arrangement, leading to a contraction. This transition is characterized by a reduction in dimensional change observed through thermomechanical analysis (TMA), as the packed PLA chains occupy less space. Cristea et al. [99] reported that the glass transition can trigger the cold crystallization, thus leading to overlapping in terms of dimensional change which results in expansion due to the softening and contraction as a result of the cold crystallization. As these processes are overlapped, it is only possible to identify the onset of the glass transition process (GT_OnSet– black arrows), and the endset of the cold crystallization (CC_EndSet– red arrows), as summarized in Table 7. As can be seen in Fig. 7, both GT_OnSet, and CC_EndSet, are moved to lower temperatures in a similar trend as observed by DSC and DMTA. Ivorra-Martinez et al. [100], have reported these overlapping phenomena in plasticized PLA with different loads of a biobased plasticizer from itaconic acid ester. They also suggested the calculation of the coefficient of linear thermal expansion (CLTE), out of these overlapping temperature range. As it can be seen in Table 7, the CLTE below the onset of the glass transition for neat PLA was 30.2 µm/(m ºC). Depending on the effectiveness of the used plasticizers, an increase in the CLTE can be obtained. As observed previously, the lowest Tg value is obtained for the EIL-plasticized PLA. Accordingly, the CLTE of this plasticized PLA formulation exhibited high values of 40.7 µm/(m ºC), which is in total agreement with the softening this plasticizer provides to PLA as previously observed by DSC and DMTA. On the other hand, ESBO-plasticized PLA gave the lowest decrease in Tg; consequently, the CLTE is the lowest among the plasticizers used in this study, with values of 35.4 µm/(m ºC). Once again, the epoxidized isobutyl soyates (EIS, and EISD), show an intermediate behaviour. This increase in the CLTE is due to the plasticizer molecules entering into the amorphous regions of PLA thus weakening the polymer-to-polymer interactions, which are responsible for the increase in dimensional change with increasing temperature [101]. Similar tendency in the CLTE values can be observed above the end of the cold crystallization, but with higher values due to the additional softening provided by the glass transition. We can clearly observe the expansion attributed to the glass transition, and the shrinkage related to the cold crystallization in plasticized PLA with ESBO and ATBC. This is because both ESBO and ATBC show a narrower temperature range for both thermal transitions. Hence, the initial expansion attributed to the softening can be clearly seen, as well as the shrinkage at higher temperatures, related to the cold crystallization. In the other formulations, both thermally-induced phenomena overlap, and it is not possible to observe neither a clear expansion related to the softening, nor a clear shrinkage related to the cold crystallization. TMA only provides the overall effects in which the expansion (due to glass transition) is counteracted by the shrinkage (due to cold crystallization).
Conclusions
Various isobutyl esters derived from soybean oil deodorizing distillate (SODD) waste, have been synthesized and subsequently subjected to an epoxidation process, achieving an epoxide oxygen content of around 4.2%. These compounds, namely epoxidized isobutyl soyates (raw – EIS, and distilled– EISD), have been proposed as environmentally plasticizers in PLA formulations, and their efficacy has been compared with epoxidized isobutyl esters of oleic acid and linoleic acid (EIO, EIL, respectively). The effectiveness of the epoxidized isobutyl soyates has been confirmed by a significant decrease in the glass transition temperature (Tg), from 61.6 ºC (neat PLA) to 48.3 ºC (PLA with 10 wt.% EIS), while even better plasticization efficiency was obtained with 10 wt.% distilled soyate (EISD), with a Tg of 44.6 ºC, slightly lower than a commonly used PLA plasticizer, namely acetyl tributyl citrate (ATBC). EIS also showed an improvement in thermal stability as SODD residues are rich in antioxidant compounds. Both epoxidized isobutyl soyates (EIS and EISD) provide an increase in elongation at break and in impact resistance up to 32 kJ/m2. Particularly noteworthy is the exceptional impact resistance improvement provided by EIO and EIL, with impact strength values of 40.0 and 50.0 kJ/m2 respectively. The morphology of fracture surfaces of formulations plasticized with SODD derivatives showed no phase separation phenomena, confirming good compatibility with the PLA matrix. Moreover, the different SODD-derived plasticizers offered a shift of the cold crystallization process towards lower temperatures, due to the increased chain mobility plasticizers provide. Overall, the results obtained open new possibilities for the utilization of SODD wastes as a raw material for the development of environmentally plasticizers for PLA.
Data Availability
No datasets were generated or analysed during the current study.
References
Das A, Ringu T, Ghosh S, Pramanik N (2023) A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym Bull 80(7):7247–7312
Nanda S, Patra BR, Patel R, Bakos J, Dalai AK (2022) Innovations in applications and prospects of bioplastics and biopolymers: a review. Environ Chem Lett 20(1):379–395
Luckachan GE, Pillai CKS (2011) Biodegradable Polymers- A Review on Recent Trends and Emerging Perspectives. J Polym Environ 19(3):637–676
Rai P, Mehrotra S, Priya S, Gnansounou E, Sharma SK (2021Apr) Recent advances in the sustainable design and applications of biodegradable polymers. Bioresource technology. 1(325)
Jiménez-Gómez CP, Cecilia JA (2020Sep 1) Chitosan: a natural biopolymer with a wide and varied range of applications. Molecules. 25(17):3981
Shaghaleh H, Xu X, Wang S (2018) Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv 8(2):825–842
Agarwal S, Singhal S, Godiya CB, Kumar S (2023) Prospects and Applications of Starch based Biopolymers. Int J Environ Anal Chem 103(18):6907–6926
Jiménez-Rosado M, Zarate-Ramírez LS, Romero A, Bengoechea C, Partal P, Guerrero A (2019) Bioplastics based on wheat gluten processed by extrusion. Journal of Cleaner Production 239
Rojas-Lema S, Nilsson K, Langton M, Trifol J, Gomez-Caturla J, Balart R, Garcia-Garcia D, Moriana R (2023) The effect of pine cone lignin on mechanical, thermal and barrier properties of faba bean protein films for packaging applications. Journal of Food Engineering 339
Nagarajan S, Radhakrishnan S, Kalkura SN, Balme S, Miele P, Bechelany M (2019) Overview of protein-based biopolymers for biomedical application. Macromolecular Chemistry and Physics 220(14):1900126
Bhaskar R, Zo SM, Narayanan KB, Purohit SD, Gupta MK, Han SS (2023) Recent development of protein-based biopolymers in food packaging applications: A review. Polymer Testing
V.C. Kalia, S.K.S. Patel, R. Shanmugam, J.K. Lee (2021) Polyhydroxyalkanoates: Trends and advances toward biotechnological applications, Bioresource Technology 326.
J. Ivorra-Martinez, M.A. Peydro, J. Gomez-Caturla, L. Sanchez-Nacher, T. Boronat, R. Balart (2023) The effects of processing parameters on mechanical properties of 3D-printed polyhydroxyalkanoates parts, Virtual and Physical Prototyping 18(1).
A. Pandey, N. Adama, K. Adjalle, J.-F. Blais (2022) Sustainable applications of polyhydroxyalkanoates in various fields: A critical review, International Journal of Biological Macromolecules 2211184–1201.
McCutcheon CJ, Zhao B, Ellison CJ, Bates FS (2021) Crazing and Toughness in Diblock Copolymer-Modified Semicrystalline Poly(L-lactide). Macromolecules 54(23):11154–11169
Kang H, Li Y, Gong M, Guo Y, Guo Z, Fang Q, Li X (2018) An environmentally sustainable plasticizer toughened polylactide. RSC Adv 8(21):11643–11651
Zhao X, Hu H, Wang X, Yu X, Zhou W, Peng S (2020) Super tough poly(lactic acid) blends: a comprehensive review. RSC Adv 10(22):13316–13368
D. Notta-Cuvier, J. Odent, R. Delille, M. Murariu, F. Lauro, J.M. Raquez, B. Bennani, P. Dubois (2014) Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties, Polymer Testing 361–9.
Maiza M, Benaniba MT, Quintard G, Massardier-Nageotte V (2015) Biobased additive plasticizing Polylactic acid (PLA). Polimeros-Ciencia E Tecnologia 25(6):581–590
Harte I, Birkinshaw C, Jones E, Kennedy J, DeBarra E (2013) The effect of citrate ester plasticizers on the thermal and mechanical properties of poly(DL-lactide). J Appl Polym Sci 127(3):1997–2003
M. Murariu, Y. Paint, O. Murariu, F. Laoutid, P. Dubois (2022) Tailoring and Long-Term Preservation of the Properties of PLA Composites with "Green" Plasticizers, Polymers 14(22).
K. Litauszki, R. Petrreny, Z. Haramania, L. Meszaros (2023) Combined effects of plasticizers and D-lactide content on the mechanical and morphological behavior of polylactic acid, Heliyon 9(4).
R. Tejada-Oliveros, J. Gomez-Caturla, O. Fenollar, N. Montanes, J. Ivorra-Martinez, D. Garcia-Garcia (2024) Assessment of non-ester monoterpenoids as biobased plasticizers for polylactide with improved ductile behaviour, Polymer 290.
Arrieta MP, Lopez J, Ferrandiz S, Peltzer MA (2013) Characterization of PLA-limonene blends for food packaging applications. Polym Testing 32(4):760–768
Gomez-Caturla J, Tejada-Oliveros R, Ivorra-Martinez J, Garcia-Sanoguera D, Balart R, Garcia-Garcia D (2024) Development and Characterization of New Environmentally Friendly Polylactide Formulations with Terpenoid-Based Plasticizers with Improved Ductility. J Polym Environ 32(2):749–762
B. Bruester, Y.-O. Adjoua, R. Dieden, P. Grysan, C.E. Federico, V. Berthe, F. Addiego (2019) Plasticization of Polylactide with Myrcene and Limonene as Bio-Based Plasticizers: Conventional vs. Reactive Extrusion, Polymers 11(8).
J. Ivorra-Martinez, J. Gomez-Caturla, N. Montanes, L. Quiles-Carrillo, F. Dominici, D. Puglia, L. Torre (2023) Effect of dibutyl itaconate on plasticization efficiency of a REX processed polylactide with peroxides, Polymer Testing 124.
J. Gomez-Caturla, I. Dominguez-Candela, M.P. Medina-Casas, J. Ivorra-Martinez, V. Moreno, R. Balart, D. Garcia-Garcia (2023) Improvement of Poly(lactide) Ductile Properties by Plasticization with Biobased Tartaric Acid Ester, Macromolecular Materials and Engineering 308(7).
R. Avolio, R. Castaldo, G. Gentile, V. Ambrogi, S. Fiori, M. Avella, M. Cocca, M.E. Errico (2015) Plasticization of poly(lactic acid) through blending, with oligomers of lactic acid: Effect of the physical aging on properties, European Polymer Journal 66533–542.
Hassouna F, Raquez J-M, Addiego F, Dubois P, Toniazzo V, Ruch D (2011) New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur Polymer J 47(11):2134–2144
D. Xie, Y. Zhao, Y. Li, A.M. LaChance, J. Lai, L. Sun, J. Chen (2019) Rheological, Thermal, and Degradation Properties of PLA/PPG Blends, Materials 12(21).
Martino VP, Ruseckaite RA, Jimenez A (2009) Ageing of poly(lactic acid) films plasticized with commercial polyadipates. Polym Int 58(4):437–444
Yang J, Pan P, Dong T, Inoue Y (2010) Crystallization kinetics and crystalline structure of biodegradable Poly(ethylene adipate). Polymer 51(3):807–815
S. Briede, A. Barkane, M. Jurinovs, V.K. Thakur, S. Gaidukovs (2022) Acrylation of biomass: A review of synthesis process: Know-how and future application directions, Current Opinion in Green and Sustainable Chemistry 35.
Wai PT, Jiang P, Shen Y, Zhang P, Gu Q, Leng Y (2019) Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv 9(65):38119–38136
L. Rios, D. Echeverri, F. Cardeno (2013) Hydroxylation of vegetable oils using acidic resins as catalysts, Industrial Crops and Products 43183–187.
J.R. Ernzen, F. Bondan, C. Luvison, C.H. Wanke, J.D.N. Martins, R. Fiorio, O. Bianchi (2016) Structure and properties relationship of melt reacted polyamide 6/malenized soybean oil, Journal of Applied Polymer Science 133(8).
Azmi IS, Jalil MJ, Hadi A (2024) Epoxidation of unsaturated fatty acid-based palm oil via peracid mechanism as an intermediate product. Biomass Conversion and Biorefinery 14(6):7847–7855
Bueno-Ferrer C, Garrigos MC, Jimenez A (2010) Characterization and thermal stability of poly(vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym Degrad Stab 95(11):2207–2212
C.V. Rajput, N.V. Sastry, N.P. Chikhaliya (2023) Phosphorous-containing flame retardant plasticizer based on<i> Cassia</i><i> fistula</i> seed oil and their application in poly(vinyl chloride) films, Industrial Crops and Products 203.
A.H. Suzuki, B.G. Botelho, L.S. Oliveira, A.S. Franca (2018) Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films, European Polymer Journal 99142–149.
I. Dominguez-Candela, J. Gomez-Caturla, S.C. Cardona, J. Lora-Garcia, V. Fombuena (2022) Novel compatibilizers and plasticizers developed from epoxidized and maleinized chia oil in composites based on PLA and chia seed flour, European Polymer Journal 173.
A. Perez-Nakai, A. Lerma-Canto, I. Dominguez-Candela, J.M. Ferri, V. Fombuena (2023) Novel Epoxidized Brazil Nut Oil as a Promising Plasticizing Agent for PLA, Polymers 15(9).
D. Garcia-Garcia, A. Carbonell-Verdu, M.P. Arrieta, J. Lopez-Martinez, M.D. Samper (2020) Improvement of PLA film ductility by plasticization with epoxidized karanja oil, Polymer Degradation and Stability 179.
A. Carbonell-Verdu, M. Dolores Samper, D. Garcia-Garcia, L. Sanchez-Nacher, R. Balart (2017) Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid), Industrial Crops and Products 104278–286.
Bouti M, Irinislimane R, Belhaneche-Bensemra N (2022) Properties investigation of epoxidized sunflower oil as bioplasticizer for poly (lactic acid). J Polym Environ 30(1):232–245
A. Orue, A. Eceiza, A. Arbelaiz (2018) Preparation and characterization of poly(lactic acid) plasticized with vegetable oils and reinforced with sisal fibers, Industrial Crops and Products 112170–180.
Kandula S, Stolp L, Grass M, Woldt B, Kodali D (2017) Functionalization of Soy Fatty Acid Alkyl Esters as Bioplasticizers. J Vinyl Add Tech 23(2):93–105
Ferri JM, Samper MD, Garcia-Sanoguera D, Reig MJ, Fenollar O, Balart R (2016) Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J Mater Sci 51(11):5356–5366
X. Liang, F. Wu, Q. Xie, Z. Wu, J. Cai, C. Zheng, J. Fu, Y. Nie (2022) Insights into biobased epoxidized fatty acid isobutyl esters from biodiesel: Preparation and application as plasticizer, Chinese Journal of Chemical Engineering 4441–50.
P.J. Asl, R. Niazmand, M. Jahani (2021) Chemical analysis of composition of raw soybean oil deodorized distillates by GC-MS, Journal of Research and Innovation in Food Science and Technology 10(1):fa1-fa10.
Khatoon S, Rajan RGR, Krishna AGG (2010) Physicochemical Characteristics and Composition of Indian Soybean Oil Deodorizer Distillate and the Recovery of Phytosterols. J Am Oil Chem Soc 87(3):321–326
L.J. Visioli, F. de Castilhos, L. Cardozo-Filho, B.T. Ferreira de Mello, C. da Silva (2016) Production of esters from soybean oil deodorizer distillate in pressurized ethanol, Fuel Processing Technology 149326–331.
Wang L, Du W, Liu D, Li L, Dai N (2006) Lipase-catalyzed biodiesel production from soybean oil deodorizer distillate with absorbent present in <i>tert</i>-butanol system. Journal of Molecular Catalysis B-Enzymatic 43(1–4):29–32
Shimada Y, Nakai S, Suenaga M, Sugihara A, Kitano M, Tominaga Y (2000) Facile purification of tocopherols from soybean oil deodorizer distillate in high yield using lipase. J Am Oil Chem Soc 77(10):1009–1013
Lv W, Wu C, Lin S, Wang X, Wang Y (2021) Integrated Utilization Strategy for Soybean Oil Deodorizer Distillate: Synergically Synthesizing Biodiesel and Recovering Bioactive Compounds by a Combined Enzymatic Process and Molecular Distillation. ACS Omega 6(13):9141–9152
Yang Z, Li H, Duan D, Yao X, Chen J, Ji H (2020) Preparation of high purity squalene from soybean oil deodorizer distillate with the combination of macroporous resin and thin-film evaporation coupling distillation. Sep Sci Technol 55(9):1611–1622
Gunawan S, Kasim NS, Ju Y-H (2008) Separation and purification of squalene from soybean oil deodorizer distillate. Sep Purif Technol 60(2):128–135
Popa O, Băbeanu NE, Popa I, Niță S, Dinu-Pârvu CE (2015) Methods for obtaining and determination of squalene from natural sources. Biomed Res Int 2015(1):367202
Kasim NS, Gunawan S, Yuliana M, Ju Y-H (2010) A simple two-step method for simultaneous isolation of tocopherols and free phytosterols from soybean oil deodorizer distillate with high purity and recovery. Sep Sci Technol 45(16):2437–2446
E. Cortés-Triviño, C. Valencia, J.M. Franco, J.M. Oliva, P. Manzanares, M.E. Eugenio, D. Ibarra (2024) Assessment of Lignin Residues from Bioethanol Production of Olive Stones as Green Chemical Thickener of Epoxidized Linseed Oil, Journal of Polymers and the Environment 1–18.
Turco R, Tesser R, Russo V, Cogliano T, Di Serio M, Santacesaria E (2021) Epoxidation of linseed oil by performic acid produced in situ. Ind Eng Chem Res 60(46):16607–16618
Nepomuceno N, Barreto V, Wellen R (2024) Effect of dicarboxylic acids’ aliphatic chain on the curing of epoxidized soybean oil (ESO) resins. J Polym Environ 32(1):45–56
Cordeiro E, Gabino AA, Henriques RR, Soares BG (2024) Polylactic acid/epoxidized linseed oil/polypropylene grafted maleic anhydride: A promising combination of toughness and rigidity. J Appl Polym Sci 141(25):e55533
D. Garcia-Garcia, A. Carbonell-Verdu, M. Arrieta, J. López-Martínez, M. Samper (2020) Improvement of PLA film ductility by plasticization with epoxidized karanja oil, Polymer Degradation and Stability 179109259.
Perez-Nakai A, Lerma-Canto A, Dominguez-Candela I, Ferri JM, Fombuena V (2023) Novel epoxidized brazil nut oil as a promising plasticizing agent for PLA. Polymers 15(9):1997
Awale RJ, Ali FB, Azmi AS, Puad NIM, Anuar H, Hassan A (2018) Enhanced flexibility of biodegradable polylactic acid/starch blends using epoxidized palm oil as plasticizer. Polymers 10(9):977
Han JZ, Zhang MC, Zhang HQ, Liu HM, Xu S (2022) Effects of modified tributyl citrate as a novel environmentally friendly plasticizer on the mechanical property and migration stability of soft polyvinyl chloride. J Vinyl Add Tech 28(4):751–761
S. Nameer, T. Deltin, P.E. Sundell, M. Johansson (2019) Bio-based multifunctional fatty acid methyl esters as reactive diluents in coil coatings, Progress in Organic Coatings 136.
Maia DLH, Fernandes FAN (2022) Influence of carboxylic acid in the production of epoxidized soybean oil by conventional and ultrasound-assisted methods. Biomass Conversion and Biorefinery 12(12):5861–5868
Zhang CQ, Ding R, Kessler MR (2014) Reduction of Epoxidized Vegetable Oils: A Novel Method to Prepare Bio-Based Polyols for Polyurethanes. Macromol Rapid Commun 35(11):1068–1074
C. Xing, L.M. Matuana (2016) Epoxidized soybean oil-plasticized poly(lactic acid) films performance as impacted by storage, Journal of Applied Polymer Science 133(12).
M.A. Pop, C. Croitoru, T. Bedo, V. Geaman, I. Radomir, M. Cosnita, S.M. Zaharia, L.A. Chicos, I. Milosan (2019) Structural changes during 3D printing of bioderived and synthetic thermoplastic materials, Journal of Applied Polymer Science 136(17).
B.W. Chieng, N.A. Ibrahim, Y.Y. Then, Y.Y. Loo (2017) Epoxidized Jatropha Oil as a Sustainable Plasticizer to Poly(lactic Acid), Polymers 9(6).
Z. Zhang, P. Jiang, D. Liu, S. Feng, P. Zhang, Y. Wang, J. Fu, H. Agus (2021) Research progress of novel bio-based plasticizers and their applications in poly (vinyl chloride), Journal of Materials Science 5610155–10182.
K. Chaochanchaikul, P. Pongmuksuwan (2021) Influence of ozonized soybean oil as a biobased plasticizer on the toughness of polylactic acid, Journal of Polymers and the Environment 1–11.
I. Dominguez-Candela, J.M. Ferri, S.C. Cardona, J. Lora, V. Fombuena (2021) Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (<i>Salvia hispanica</i> L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid), Polymers 13(8).
Ali F, Chang YW, Kang SC, Yoon JY (2009) Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym Bull 62(1):91–98
J. Sempere-Torregrosa, J.M. Ferri, H. de la Rosa-Ramírez, C. Pavon, M.D. Samper (2022) Effect of Epoxidized and Maleinized Corn Oil on Properties of Polylactic Acid (PLA) and Polyhydroxybutyrate (PHB) Blend, Polymers 14(19).
Zych A, Perotto G, Trojanowska D, Tedeschi G, Bertolacci L, Francini N, Athanassiou A (2021) Super Tough Polylactic Acid Plasticized with Epoxidized Soybean Oil Methyl Ester for Flexible Food Packaging. Acs Applied Polymer Materials 3(10):5087–5095
Maiza M, Benaniba MT, Massardier-Nageotte V (2016) Plasticizing effects of citrate esters on properties of poly(lactic acid). J Polym Eng 36(4):371–380
Vieira MGA, da Silva MA, dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: A review. Eur Polymer J 47(3):254–263
Ke TY, Sun XZ (2003) Melting behavior and crystallization kinetics of starch and poly(lactic acid) composites. J Appl Polym Sci 89(5):1203–1210
Zhang J, Tashiro K, Tsuji H, Domb AJ (2008) Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 41(4):1352–1357
Dutta K, Das S, Kundu PP (2014) Epoxidized Esters of Palm Kernel Oil as an Effective Plasticizer for PVC: A Study of Mechanical Properties and Effect of Processing Conditions. Int Polym Proc 29(4):495–506
H. Hosney, B. Nadiem, I. Ashour, I. Mustafa, A. El-Shibiny (2018) Epoxidized vegetable oil and bio-based materials as PVC plasticizer, Journal of Applied Polymer Science 135(20).
Fenollar O, Garcia-Sanoguera D, Sanchez-Nacher L, Boronat T, López J, Balart R (2013) Mechanical and Thermal Properties of Polyvinyl Chloride Plasticized with Natural Fatty Acid Esters. Polym-Plast Technol Eng 52(8):761–767
Al-Mulla EAJ, Yunus W, Ibrahim NAB, Ab Rahman MZ (2010) Properties of epoxidized palm oil plasticized polytlactic acid. J Mater Sci 45(7):1942–1946
L. Quiles-Carrillo, S. Duart, N. Montanes, S. Torres-Giner, R. Balart (2018) Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil, Materials & Design 14054–63.
Sustaita-Rodríguez A, Vega-Rios A, Bugarin A, Ramos-Sánchez VH, Camacho-Dávila AA, Rocha-Gutiérrez B, Chávez-Flores D (2021) Chemoenzymatic Epoxidation of Highly Unsaturated Fatty Acid Methyl Ester and Its Application as Poly(lactic acid) Plasticizer. Acs Sustainable Chemistry & Engineering 9(50):17016–17024
Liu W, Fu XL, Li ZZ (2019) Extraction of Tocopherol from Soybean Oil Deodorizer Distillate by Deep Eutectic Solvents. J Oleo Sci 68(10):951–958
Tian JR, Cao ZQ, Qian SP, Xia YB, Zhang JX, Kong YQ, Sheng KC, Zhang Y, Wan Y, Takahashi J (2022) Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose. Nanotechnol Rev 11(1):2469–2482
T. Tábi, T. Ageyeva, J.G. Kovács (2021) Improving the ductility and heat deflection temperature of injection molded Poly(lactic acid) products: A comprehensive review, Polymer Testing 101.
Baiardo M, Frisoni G, Scandola M, Rimelen M, Lips D, Ruffieux K, Wintermantel E (2003) Thermal and mechanical properties of plasticized poly(L-lactic acid). J Appl Polym Sci 90(7):1731–1738
Piorkowska E, Kulinski Z, Galeski A, Masirek R (2006) Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 47(20):7178–7188
Y.B. Tee, R.A. Talib, K. Abdan, N.L. Chin, R.K. Basha, K.F.M. Yunos, Toughening Poly(lactic acid) and Aiding the Melt-compounding with Bio-sourced Plasticizers, 2nd International Conference on Agricultural and Food Engineering (CAFE), Kuala Lumpur, MALAYSIA, 2014, pp. 289–295.
Ljungberg N, Wesslén B (2002) The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J Appl Polym Sci 86(5):1227–1234
Ljungberg N, Wesslén B (2005) Preparation and properties of plasticized poly(lactic acid) films. Biomacromol 6(3):1789–1796
M. Cristea, D. Ionita, M.M. Iftime (2020) Dynamic Mechanical Analysis Investigations of PLA-Based Renewable Materials: How Are They Useful?, Materials 13(22).
J. Ivorra-Martinez, M.A. Peydro, J. Gomez-Caturla, T. Boronat, R. Balart (2022) The Potential of an Itaconic Acid Diester as Environmentally Friendly Plasticizer for Injection-Molded Polylactide Parts, Macromolecular Materials and Engineering 307(12).
J.F. Balart, V. Fombuena, O. Fenollar, T. Boronat, L. Sánchez-Nacher (2016) Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO), Composites Part B-Engineering 86168–177.
Acknowledgements
This research is a part of the grant PID2023-152869OB-C22, funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and the grant TED2021-131762A-I00, funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by the European Union “NextGenerationEU”/PRTR. Authors also thank Generalitat Valenciana-GVA for funding this research through the grant numbers AICO/2021/025 and CIGE/2021/094. J. Gomez-Caturla wants to thank FPU20/01732 grant funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by ESF Investing in your future.
Funding
Agencia Estatal de Investigación,TED2021-131762A-I00,TED2021-131762A-I00,TED2021-131762A-I00,TED2021-131762A-I00,TED2021-131762A-I00,TED2021-131762A-I00,Generalitat Valenciana,AICO/2021/025,AICO/2021/025,AICO/2021/025,AICO/2021/025,AICO/2021/025, AICO/2021/025. Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
L. N-L. wrote the draft of the manuscript and conducted the experimental work, P.C.N-R. and J.G-C. revised the manuscript and edited it for publication, A.O. also conducted the experimental work and prepared the figures of the manuscript, O.F. and R.B. obtained funding and supervised the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Najera-Losada, L., Narváez-Rincón, P.C., Orjuela, A. et al. Plasticization of Polylactide Using Biobased Epoxidized Isobutyl Esters Derived from Waste Soybean Oil Deodorizer Distillate. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03415-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03415-1