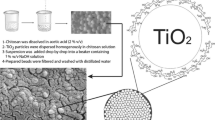
Abstract
This study developed three microstructured chitosan nanocapsules with immobilized lipase to explore chitosan-lipase interactions at different pH levels. Chitosan undergoes complete protonation or deprotonation based on pH level. Three distinct pH levels were examined: 5.5, where chitosan is fully protonated; 6.5, where chitosan is partially protonated/deprotonated; and 7.5, where chitosan is fully deprotonated. The nanocapsules exhibited nanoscale dimensions and the microstructures showed porous morphology. Immobilized lipase showed improved temperature stability, compared to free enzyme, especially in lipase supports at pH 5.5 and 7.5 due to electrostatic and hydrophobic interactions. The interactions between chitosan and lipase influenced the microenvironment around the active site, resulting in an optimum pH of 8 for all supports. Immobilized lipase at pH 5.5 and 7.5 displayed the best reusability in the hydrolysis of p-nitrophenyl palmitate under reaction conditions of 37 °C and pH 8. During refrigeration storage, all immobilized lipases maintained total activity for 7 days, but lipase immobilized at pH 6.5 maintained more the activity after 28 days. Therefore, this study has developed promising immobilized lipase, standing out not only for industrial application concerning cost-effectiveness, but also for the innovation in investigating the influence of chitosan-lipase interactions during immobilization.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ueda M, Takahashi S, Washida M et al (2002) Expression of Rhizopus oryzae lipase gene in Saccharomyces cerevisiae. J Mol Catal B Enzym 17:113–124. https://doi.org/10.1016/S1381-1177(02)00018-8
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235. https://doi.org/10.1039/C3CS60075K
Maghraby YR, El-Shabasy RM, Ibrahim AH, Azzazy HMES (2023) Enzyme immobilization technologies and industrial applications. ACS Omega 8:5184–5196. https://doi.org/10.1021/acsomega.2c07560
Ribeiro ES, de Farias BS, Sant’Anna Cadaval Junior TR et al (2021) Chitosan–based nanofibers for enzyme immobilization. Int J Biol Macromol 183:1959–1970. https://doi.org/10.1016/j.ijbiomac.2021.05.214
Zhong L, Feng Y, Wang G et al (2020) Production and use of immobilized lipases in/on nanomaterials: a review from the waste to biodiesel production. Int J Biol Macromol 152:207–222. https://doi.org/10.1016/j.ijbiomac.2020.02.258
Mondal S, Alke B, de Castro AM et al (2022) Design of enzyme loaded W/O emulsions by direct membrane emulsification for CO2 capture. Membr (Basel) 12:797. https://doi.org/10.3390/membranes12080797
Zhang L, Zhang F, Fan Z et al (2019) DHA and EPA nanoemulsions prepared by the low-energy emulsification method: process factors influencing droplet size and physicochemical stability. Food Res Int 121:359–366. https://doi.org/10.1016/j.foodres.2019.03.059
Tang C, Chai Y, Wang C et al (2022) Pickering emulsions stabilized by lignin/chitosan nanoparticles for biphasic enzyme catalysis. Langmuir 38:12849–12858. https://doi.org/10.1021/acs.langmuir.2c01819
Meng W, Sun H, Mu T, Garcia-Vaquero M (2023) Chitosan-based Pickering emulsion: a comprehensive review on their stabilizers, bioavailability, applications and regulations. Carbohydr Polym 304:120491. https://doi.org/10.1016/j.carbpol.2022.120491
Roda Zitha Vilanculos J, Silva de Farias B, Inês Engelmann J et al (2023) Physicochemical evaluation of chitosan–xanthan gum nanoemulsions as polyunsaturated enriched lipid–carrier. J Mol Liq 386:122533. https://doi.org/10.1016/J.MOLLIQ.2023.122533
Engelmann JI, de Farias BS, Igansi AV et al (2023) Chitosan-based nanocapsules by emulsification containing PUFA concentrates from tuna oil. Food Sci Technol Int. https://doi.org/10.1177/10820132231153496
Cao M, Cao A, Xing J et al (2023) Pickering emulsion stabilized by parasin I and chitosan nanoparticles enhances protection against intestinal microbiota homeostasis by reducing inflammation in peritonitis mice. Int J Biol Macromol 242:125016. https://doi.org/10.1016/J.IJBIOMAC.2023.125016
Song S, Zhong L, Wei Y et al (2023) Highly stable solid-like Pickering emulsions stabilized by kafirin-chitosan complex particles. LWT 177:114591. https://doi.org/10.1016/j.lwt.2023.114591
Liu Y-W, Li Q-H, Li S-Y et al (2023) Interfacial adsorption behavior of the aspergillus oryzae lipase-chitosan complex and stability evaluation of the resultant Pickering emulsion. Int J Biol Macromol 233:123599. https://doi.org/10.1016/j.ijbiomac.2023.123599
de Farias BS, Sant’Anna Cadaval Junior TR, de Almeida Pinto LA (2019) Chitosan-functionalized nanofibers: a comprehensive review on challenges and prospects for food applications. Int J Biol Macromol 123:210–220. https://doi.org/10.1016/j.ijbiomac.2018.11.042
Sharkawy A, Barreiro MF, Rodrigues AE (2020) Chitosan-based Pickering emulsions and their applications: a review. Carbohydr Polym 250:116885. https://doi.org/10.1016/j.carbpol.2020.116885
Weng M, Xia C, Xu S et al (2022) Lipase/chitosan nanoparticle-stabilized pickering emulsion for enzyme catalysis. Colloid Polym Sci 300:41–50. https://doi.org/10.1007/s00396-021-04923-5
Weska RF, Moura JM, Batista LM et al (2007) Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J Food Eng 80:749–753. https://doi.org/10.1016/j.jfoodeng.2006.02.006
Moura JM, Farias BS, Rodrigues DAS et al (2015) Preparation of Chitosan with different characteristics and its application for biofilms production. J Polym Environ 23:470–477. https://doi.org/10.1007/s10924-015-0730-y
Dotto GL, de Souza VC, de Moura JM et al (2011) Influence of drying techniques on the characteristics of chitosan and the quality of biopolymer films. Drying Technol 29:1784–1791. https://doi.org/10.1080/07373937.2011.602812
Jiang X, Chen L, Zhong W (2003) A new linear potentiometric titration method for the determination of deacetylation degree of Chitosan. Carbohydr Polym 54:457–463. https://doi.org/10.1016/J.CARBPOL.2003.05.004
Masuelli MA (2014) Mark-Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. J Polym Biopolymer Phys Chem 2:37–43. https://doi.org/10.12691/jpbpc-2-2-2
Goldstein JI, Newbury DE, Echlin P et al (2003) Special topics in electron beam X-Ray microanalysis. Scanning Electron Microscopy and X-ray Microanalysis. Springer US, Boston, MA, pp 453–536
Silverstein RM, Bassler GC (1962) Spectrometric identification of organic compounds. J Chem Educ 39:546. https://doi.org/10.1021/ed039p546
de Farias BS, Grundmann DDR, Rizzi FZ et al (2019) Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res Int 123:88–94. https://doi.org/10.1016/j.foodres.2019.04.051
Maia M (2001) Effect of culture conditions on lipase production by Fusarium solani in batch fermentation. Bioresour Technol 76:23–27. https://doi.org/10.1016/S0960-8524(00)00079-1
Felder R, Richard M, Ronald W et al (2020) Elementary principles of Chemical processes. Jhon Wyley and Sons, New York, pp 1–668
Moraes PS, Engelmann JI, Igansi AV et al (2021) Nile tilapia industrialization waste: evaluation of the yield, quality and cost of the biodiesel production process. J Clean Prod 287:125041. https://doi.org/10.1016/j.jclepro.2020.125041
Liu YW, Zhou Y, Huang GQ et al (2022) Fabrication of lipase-loaded particles by coacervation with chitosan. Food Chem 385:132698. https://doi.org/10.1016/j.foodchem.2022.132689
Namboodiri VMH, Chattopadhyaya R (2000) Purification and biochemical characterization of a novel thermostable lipase from Aspergillus Niger. Lipids 35:495–502. https://doi.org/10.1007/s11745-000-549-3
Liu H, Wang C, Zou S et al (2012) Simple, reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification. Langmuir 28:11017–11024. https://doi.org/10.1021/la3021113
Sarkar A, Kellogg G (2010) Hydrophobicity - Shake flasks, protein folding and drug discovery. Curr Top Med Chem 10:67–83. https://doi.org/10.2174/156802610790232233
Mazzobre MF, Longinotti MP, Corti HR, Buera MP (2001) Effect of salts on the properties of aqueous sugar systems, in relation to biomaterial stabilization. 1. Water sorption behavior and ice crystallization/melting. Cryobiology 43:199–210. https://doi.org/10.1006/cryo.2001.2345
Sun L, Zhu Z, Sun DW (2023) Regulating ice formation for enhancing frozen food quality: materials, mechanisms and challenges. Trends Food Sci Technol 139:104116. https://doi.org/10.1016/j.tifs.2023.07.013
Jegannathan KR, Jun-Yee L, Chan E-S, Ravindra P (2010) Production of biodiesel from palm oil using liquid core lipase encapsulated in κ-carrageenan. Fuel 89:2272–2277. https://doi.org/10.1016/j.fuel.2010.03.016
Jegannathan KR, Chan E-S, Ravindra P (2009) Physical and stability characteristics of Burkholderia cepacia lipase encapsulated in κ-carrageenan. J Mol Catal B Enzym 58:78–83. https://doi.org/10.1016/j.molcatb.2008.11.009
Huang X-J, Ge D, Xu Z-K (2007) Preparation and characterization of stable chitosan nanofibrous membrane for lipase immobilization. Eur Polym J 43:3710–3718. https://doi.org/10.1016/j.eurpolymj.2007.06.010
Ye P, Xu Z-K, Wu J et al (2006) Nanofibrous poly(acrylonitrile-co-maleic acid) membranes functionalized with gelatin and chitosan for lipase immobilization. Biomaterials 27:4169–4176. https://doi.org/10.1016/j.biomaterials.2006.03.027
Barriuso J, Vaquero ME, Prieto A, Martínez MJ (2016) Structural traits and catalytic versatility of the lipases from the Candida rugosa-Like family: a review. Biotechnol Adv 34:874–885. https://doi.org/10.1016/j.biotechadv.2016.05.004
Ye P, Xu Z, Che A et al (2005) Chitosan-tethered poly(acrylonitrile–maleic acid) hollow fiber membrane for lipase immobilization. Biomaterials 26:6394–6403. https://doi.org/10.1016/j.biomaterials.2005.04.019
Yadav GD, Jadhav SR (2005) Synthesis of reusable lipases by immobilization on hexagonal mesoporous silica and encapsulation in calcium alginate: transesterification in non-aqueous medium. Microporous Mesoporous Mater 86:215–222. https://doi.org/10.1016/j.micromeso.2005.07.018
Huang X-J, Chen P-C, Huang F et al (2011) Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J Mol Catal B Enzym 70:95–100. https://doi.org/10.1016/j.molcatb.2011.02.010
Acknowledgements
The authors would like also to thank Nanosul/FURG of the Associate Laboratory of the National System of Laboratories in Nanotechnology (SisNANO)/Brazil, Center for Electron Microscopy of the South Zone (CEME-SUL)/FURG/Brazil, and Integrated Analysis Center of the Federal University of Rio Grande (CIA)/FURG/Brazil for research support.
Funding
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brazil - Finance Code 001, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)/Brazil, the Fundação de Amparo à Pesquisa do Estado do RS (FAPERGS)/Brazil, the Secretaria de Desenvolvimento, Ciência e Tecnologia/RS/Brazil (projects DCIT 70/2015 and DCIT 77/2016) for the financial support.
Author information
Authors and Affiliations
Contributions
E. S. R.: Conceptualization, Investigation, Formal analysis, Writing—Review and editing, Data curation. B. R. M.: Investigation, Formal analysis. B. S. F.: Conceptualization, Investigation, Formal analysis, Writing—Review and editing, Data curation. L. O. S.: Project administration, Funding acquisition. S. H. D.: Project administration, Funding acquisition. T. R. S. C. Jr: Project administration, Funding acquisition. L. A. A. P.: Project administration, Funding acquisition. Patrícia Silva Diaz: Conceptualization, visualization, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ribeiro, E.S., Machado, B.R., de Farias, B.S. et al. Development of Microstructured Chitosan Nanocapsules with Immobilized Lipase. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03187-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03187-8