Abstract
In this study, a new polymeric oleic acid-derived macro addition/fragmentation transfer agent was utilized to produce a poly(styrene)-g-poly(oleic acid) graft copolymer. The double bond of oleic acid was initially saturated with bromine and the condensation polymerization between the carboxylic acid and the bromide resulted in polyoleic acid with pendant bromide groups. Xanthate groups were exchanged with the bromide groups to obtain the poly(oleic acid) macro RAFT agent (Pole-Xa). Poly(styrene)-g-poly(oleic acid) (PS-g-Pole) graft copolymers were synthesized via reversible addition fragmentation transfer (RAFT) polymerization of styrene and the reaction was evaluated in view of the polymerization kinetics. The effects of polymerization temperature and reaction time on graft copolymer yield, conversion and molecular weight were investigated. In the RAFT polymerization of styrene, the rate constant (k) was found to be 1.83 × 10–3 L/mol/dk and 7.27 × 10–4 L/mol/dk for the polymerization temperatures of 80 and 90 °C, respectively. The structural characteristics and thermal properties of the obtained products were characterized using FT-IR, 1H-NMR, GPC, TGA, DSC and SEM–EDX.
Similar content being viewed by others
Introduction
Controlled/living polymerization techniques such as nitroxide-mediated radical polymerization (NMP) [1,2,3], atom transfer radical polymerization (ATRP) [4,5,6,7,8,9], reversible addition fragmentation transfer (RAFT) polymerization and anionic polymerization are established techniques for copolymer synthesis. Among these techniques, RAFT, which was first introduced by Rizzardo et al. [10] and based on radical polymerization, has been successfully utilized in the synthesis of many types of block/graft copolymers due to the easy polymerization conditions using various chain transfer agents (CTA). In RAFT polymerization, the control of polymer molecular weight is provided by the specific chain transfer agents (CTA) used in the reaction. Xanthates are the preferred CTA agents because they are inexpensive and readily available. In this study, xanthate-mediated RAFT polymerization was utilized because of its easy applicability and mild polymerization conditions. [10,11,12,13,14,15,16,17,18,19,20,21,22,23].
Vegetable oil-based polymers (VOP) are attractive among biomaterials because they are derived from renewable resources that are inherently biodegradable and show low toxicity. Typically VOP are synthesized by taking advantage of the carbon–carbon double bonds associated with the individual fatty acids that make up the oil or in certain circumstances the functional groups associated with the fatty acids. These may include hydroxyl groups as in ricinoleic acid or lesquerolic acid or epoxy groups as in vernolic acid. Depending on the reaction conditions, VOP have been synthesized with a variety of material properties ranging from soft and flexible rubbers to hard and rigid thermoplastics [24]. Oleic acid is one fatty acid that is found naturally and abundantly in various animal fats and vegetable oils. It is an odorless, colorless oil that is chemically classified as a monounsaturated omega-9 fatty acid that, because of its acrylic double bond, can be utilized as an important raw material for obtaining active-ended chemicals. It can be easily reacted to yield polymeric macroinitiators which can then be used to synthesize block/graft copolymers by connecting different monomers to their active sites due to the active centers at the chain ends of polymeric macroinitiators [25,26,27,28,29,30,31,32,33,34].
The importance of graft copolymers is gradually increasing, with numerous studies reporting from synthesis to application every year. Graft copolymers have been extensively studied due to their intriguing properties that are inherently different compared to their linear counterparts, and their importance as a macromolecular structure has been highlighted [35, 36]. The methods of preparation of such polymers are of interest because the properties of graft copolymers are different from the homopolymers of the monomers from which they are formed. Although a graft copolymer obtained from a single polymerization method is known to allow for facile synthesis, the combination of two different polymerization techniques can be an advantageous way to incorporate classes of monomers that may be incompatible [8, 27, 37].
In this study, graft copolymers were prepared by grafting PS onto the active centers of the Pole chains using two different techniques such as condensation and RAFT polymerization. Macropolymeric oils were synthesized by condensation polymerization to obtain graft copolymers via RAFT polymerization. The basis of this study was to prepare oil polymers, which are a natural product, and to report the synthesis of new partially biodegradable polymers by attaching a naturally biodegradable oil polymer to vinyl polymer such as polystyrene (PS) using graft copolymer synthesis.
Polystyrene (PS) is a petroleum-based polymer produced by the polymerization of styrene. At room temperature polystyrene is a solid thermoplastic with a high melting temperature (~ 240 °C) when processed by injection or extrusion. There have been many well-known polystyrene-containing block/graft copolymers in the literature. For example, Tuzen et al. [38] obtained and evaluated poly(styrene)-co-2-vinylpyridine by the reversible addition fragmentation chain transfer (RAFT) polymerization of styrene and 2-vinyldene. Rajendran et al. [39] using a styrene-based macro-RAFT agent, produced polystyrene-graft-polymethyl methacrylate (PS-g-PMMA), polystyrene-graft-poly(isobornyl acrylate), polystyrene-graft-poly[2(acetoacetoxy)ethyl methacrylate] (PS-g-PAEMA), and poly(paramethoxystyrene)-graft-polystyrene(P(p-MS)-g-PS) copolymers. Khani et al. [40] investigated RAFT preparation of well-defined polyisoprene grafted silica nanoparticles (PIP-g-SiO2NPs) while Bolton et al. [41] reported the synthesis of a poly(styrene)-b-poly(methyl methacrylate) (PS-PMMA) bottlebrush block copolymer with asymmetrical branches by RAFT and ATRP polymerizations. Hazer et al. [42] reported a range of polymers produced by RAFT polymerization of styrene (St), vinyl benzyl chloride (vbc), tertbutyl methacrylate (tertBMA), and n-butyl methacrylate (nBMA) using carboxylic acid functionalized trithiocarbonate. Göktaş [43] synthesized poly(methyl methacrylate-b-styrene) and poly(methyl methacrylate-b-acrylamide) block copolymers in two steps via a redox polymerization method coupled with an atom transfer radical polymerization (ATRP) method. Lastly, Öztürk et al. [44] synthesized triarm block copolymers containing one polystyrene (or polyacrylamide) arm and two poly(β-butyrolactone) arms in one step by simultaneous use of RAFT polymerization of styrene (St) (or acrylamide, designated as AAm) and ROP of β-butyrolactone (BL) [45,46,47,48].
In this contribution, further utilization of RAFT polymerization is described utilizing brominated polyoleic acid (Pole-Br) synthesized by condensation polymerization. This Pole-Br was used as a suitable initiator for controlled/living radical polymerizations by acting as a novel macro RAFT agent in the exchange of the bromide groups of polyoleic acid with potassium ethyl xanthate resulting in poly(styrene)-graft-poly(oleic acid) graft copolymers. To our knowledge, PS-g-Pole graft copolymers will be brought to the literature with this study by combining RAFT and condensation polymerizations.
Experimental
Chemicals
Potassium ethyl xanthogenate salt (KXa), 2,2’-azobisisobutyronitrile (AIBN), oleic acid, styrene (S), tetrahydrofuran (THF), and N,N-dimethylformamide (DMF) were supplied by Aldrich and used as received. Methanol, chloroform, petroleum ether, K2CO3, Br2, and Na2SO4, were supplied by Fluka and used as received.
Synthesis of Polymeric Brominated Oleic Acid (Pole-Br) by Condensation Polymerization
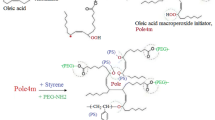
2.7 g of Br2, a 10 g solution of Br2/CCl4 (1:1) and 12 g of oleic acid solution [(CHS) oleic acid: 5.06 g/15 g CCl4] were added into a glass flask and stirred in a water bath at room temperature for 24 h. The product was then poured into excess distilled water. The brominated oleic acid (ole-Br) was washed an additional two times with distilled water. The oily phase was separated from the water phase and dried using anhydrous Na2SO4. The yield for the obtained product was 7.18 g. The bromination of the oleic acid is shown in Scheme 1A. Referring to the condensation polymerization mechanism, specified amounts of ole-Br, K2CO3 and DMF (Table 1) were added to a flask and stirred at 85 °C for 24 h resulting in polymeric brominated oleic acid (Pole-Br; Scheme 1B).
Synthesis of a Novel Oleic Acid-Based Macro RAFT Agent (Pole-Xa)
To synthesize the poly(oleic acid) macro RAFT agent (Pole-Xa), 1.12 g of polymeric brominated oleic acid (Pole-Br), 1.76 g of potassium ethyl xanthogenate (KXa) and 40 mL of THF were combined and mixed using a magnetic stirrer at room temperature for 72 h. The formed KBr and excess KXa salts were removed by filtration. The solvent was evaporated with a rotary evaporator and the product was washed with water, then with methanol and dried at room temperature for one week and subsequently under vacuum at 50 °C for 1 day. The chemical synthesis reaction of the macro-RAFT agent (Pole-Xa) is shown in Scheme 1C.
Synthesis of Copolymers by RAFT Polymerization
In accordance with the RAFT mechanism, various amounts of Styrene (S), 2,2'-azobisisobutyronitrile (AIBN) and RAFT agent (Pole-Xa) were placed in a Schlenk tube and allowed to form a homogeneous solution. The specific amounts of each chemical used in the polymerization reactions are given in detail in Table 2 and 3. Argon was passed through the solution. After closing the tube, polymerization was carried out in a silicone oil bath at 80 and 90 °C for between 1 and 5 h. At the end of the polymerization reaction, the contents of the tube were poured into excess petroleum ether and the poly(styrene)-g-poly(oleic acid) [(PS-g-Pole)] graft copolymer was precipitated and dried under vacuum for one week. The synthesis mechanism of PS-g-Pole graft copolymers is as shown in Scheme 1D.
Analysis
Molecular Weight Determination
The molecular weights and molecular weight distributions of the synthesized polymers were determined by gel-permeation chromatography (GPC) using a Malvern Viscotek GPCmax device with THF as the mobile phase. Each of the samples was weighed (~ 25 mg) and dissolved in 10 mL of THF. The dissolved samples were transferred to separate vials after passing each through a 0.22 PTFE syringe filter. The conditions of each analysis were as follows: (1) Detector: VE 3580 RI detector, (2) TGuard + 2xT6000M column, (3) Solvent: THF, (4) Detector Temperature: 35 °C, (5) Column Temperature: 35 °C, (6) Injection volume: 100 µL, (7) Flow rate: 1 mL/min, (8) Analysis time: 40 min/per sample. A polystyrene (PS) calibration curve with 12 standards ranging from 1000 Da to 4.5 MDa was used for post-analysis calculations.
Composition Determination
Fourier transform infrared spectroscopy (FTIR) was utilized to determine the functional groups associated with the chemical intermediates (initiators) and final graft copolymers. The spectra were recorded with a Perkin Elmer (Shelton, CT USA) Spectrum 100 spectrometer in transmit mode with a scanning speed of 4000–550 cm−1. Structural characterization of the synthesized initiators and the graft copolymers was performed by 1H-nuclear magnetic resonance (1H-NMR) spectroscopy on a Bruker Ultra Shield Plus, ultra-long retention time 400 MHz spectrometer using deuterated chloroform as solvent.
Thermal Property Determination
Thermal analyses of the synthesized polymers were performed using the Lab SYS EVO TGA/DSC thermo gravimetric analysis (TGA) device and differential scanning calorimetry (DSC) with the heating rate of 10 °C/min under Argon gas protection. The TGA temperature range was from 25 to 550 °C while DSC (calibrated with indium; Tm = 156.6 °C) was performed using a temperature range between 25 and 200 °C.
Results and Discussion
Synthesis of Polymeric Brominated Oleic Acid (Pole-Br) by Condensation Polymerization
In order to follow any chemical reaction it is imperative to properly analyze and understand the characteristics of the starting materials. In this study, oleic acid was utilized as the precursor for the synthesis of brominated oleic acid which was subsequently used in the formation of polymeric brominated oleic acid (Pole-Br) by condensation polymerization. The 1H-NMR scan of oleic acid (Fig. 1a) ascribes the protons associated with the terminal CH3 group at 0.8 ppm, the protons associated with the internal CH2 groups at 1.2 and 2.4 ppm, and the proton associated with the methine groups (CH) involved with the olefinic carbons at 5.6 ppm. Bromination and polymerization resulted in Pole-Br whose 1H-NMR results are shown in Fig. 1b. By comparison, the resonance present at 5.6 ppm in the oleic acid result disappeared while a separate resonance at 4.2 ppm corresponding to CH-Br developed. This shift demonstrates the conversion of the olefinic groups in oleic acid to brominated carbons within the Pole-Br monomers. The FT-IR spectrum of the Pole-Br shows a C–Br band at 722 cm−1, C=O band at 1720 cm−1, an aliphatic CH band at 2920 cm−1 and an OH band at 3500 cm−1 which helped confirm the reaction (Fig. 2a). The molecular weight of the synthesized Pole-Br was 18,100 g/mol (number average molecular weight; Mn) with a polydispersity (Đ; Mw/Mn) of 2.05 (Table 1).
Synthesis of a Novel Oleic Acid-Based Macro RAFT Agent (Pole-Xa)
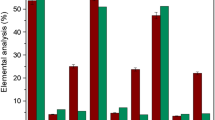
The Pole-Br (synthesis was described in “Synthesis of Polymeric Brominated Oleic Acid (Pole-Br) by Condensation Polymerization” Sect.), was subsequently used to produce the poly(oleic acid) macro RAFT agent (Pole-Xa) for use in the xanthate-mediated RAFT polymerization reaction. Successful synthesis of Pole-Xa was confirmed by 1H-NMR (Fig. 1c) and FT-IR (Fig. 2b) of the final product. NMR resonances at 0.8 ppm (CH3), 1.2 and 2.4 ppm (CH2), 3.5 ppm (OCH2), and 4.3 (CH-S) inferred the successful production of Pole-Xa. This result was further confirmed through FT-IR spectroscopy of the macro RAFT agent (Pole-Xa). Bands associated with the C=S group at 1600 cm−1, the C=O group at 1720 cm−1, an aliphatic CH group at 2852–2923 cm−1 and OH bands at 3500 cm−1 were present signifying the successful conversion of Pole-Br to Pole-Xa which was then used in the reaction to produce the poly(styrene-graft-oleic acid) copolymers (PS-g-Pole) through RAFT polymerization. The SEM and EDX analysis of macro RAFT agent (Pole-Xa) is given in Fig. 3. The molecular weight of Pole-Xa determined by GPC (Mn) was 18,400 g/mol.
Synthesis of the Poly(Styrene-Graft-Oleic Acid) Copolymers by RAFT Polymerization
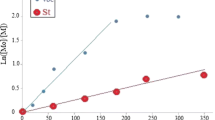
RAFT polymerization of styrene (S) was performed at either 80 or 90 °C via a novel macro RAFT agent (Pole-Xa) obtained from the reaction of potassium ethyl xanthate (KXa) and Pole-Br resulting in poly(styrene-graft-oleic acid) copolymers (PS-g-Pole). For the first time in this study, PS-g-Pole graft copolymers were introduced to the macromolecular library with this RAFT polymerization in the presence of a polyoleic-based macro RAFT agent synthesized by condensation polymerization (described previously). The suitability of the Pole-Xa for use in the solvent-free xanthate-mediated RAFT polymerization of styrene was also reported for the first time with this paper. The 1H-NMR of the PS-g-Pole graft copolymer show resonances at 0.9 ppm (CH3), 1.2 ppm (CH2) which correspond to the Pole block of the copolymer and the PS block of the graft copolymers, and at 1.8 ppm (CH) which correspond to the PS block of the graft copolymers. Additionally, the protons associated with the phenyl groups were detected at between 6.6 and 7.2 ppm (Fig. 1d). In the FT-IR spectrum of the PS-g-Pole graft copolymer, bands corresponding to aliphatic CH (2852–2923 cm−1) and aromatic CH (3025 cm−1) were shown (Fig. 2c). The effects of polymerization time and temperature on polymer molecular weight and yield are shown in Table 2 and 3. Generally, the molecular weights of the synthesized PS-g-Pole graft copolymers were higher at identical polymerization times when produced at 80 °C over those produced at 90 °C. However, as expected, at each temperature increased polymerization times resulted in larger molecular weights until a threshold polymerization time was reached where the molecular weights stabilized (Figs. 4 and 5). At 80 °C, as the polymerization times increased in 1-h increments, the Mn values of the graft copolymers increased by an average of 8.6 ± 2.9% per hour up to 4 h reaction time. Between 4 and 5 h the Mn stabilized and in fact, decreased slightly by 1.8%. In contrast, at 90 °C the Mn values of the graft copolymers were on average 23.4 ± 6.0% smaller than the graft copolymers produced at 80 °C at the same polymerization times. In addition, the maximum Mn value for the graft copolymers produced at 90 °C occurred at 3 h instead of 4 h (as at 80 °C) and the Mw/Mn for each of the graft copolymers produced at 90 °C (with the exception of the polymers produced at 1-h), were above 2.00. By comparison, the Mw/Mn values of the polymers produced at 80 °C were between 1.63 and 1.91. The reason why the Mw/Mn values were higher than expected is due to the high molecular weight of the initiator used in the polymerization and the presence of many active centers in the polymerization environment (Table 2 and 3). This phenomenon has been reported in our previous articles [18,19,20].
The effect of 80 °C polymerization temperature and polymerization time on molecular weight and conversion (Table 2)
The effect of 90 °C polymerization temperature and polymerization time on molecular weight and conversion (Table 3)
Conversion represents the amount of graft copolymer that was produced from a given amount of reactants. In this study at 80 °C the yields (g) and conversion (%) increased from 0.70 to 2.26 g and from 13.48 to 43.31% (Fig. 4; Table 2), respectively as the reaction times increased. At 90 °C the yield and conversion values were approximately 100% higher than those at 80 °C after 1 h reaction time but ultimately reached a comparative maximum (yield = 2.26 g; conversion = 43.16%) 1 h earlier than at 80 °C (Fig. 5; Table 3). This indicates that at 90 °C the reaction initiates quicker and terminates sooner but ultimately, the maximum yields and conversions remained comparable.
Using the assumption that a RAFT agent compound forms a polymer chain in controlled/living radical polymerization methods, the theoretical molecular weight (Mn,theo) is calculated according to Eq. 1 [49]; where [M]o and [RAFT-agent]o are the initial concentrations of monomer and RAFT agent, (Mmon)o represents the molar weight of the monomer, and 18,400 represents the molar weight of the RAFT agent. The theoretical molecular weight (Mn,theo) calculated according to Eq. 1 is shown in Tables 2 and 3.
The weight percentages of each block in the structure of the graft copolymers were also determined by calculating the peak areas of methyl protons in Pole (0.8 ppm) and phenyl protons in polystyrene (6.6–7.2 ppm) in 1H-NMR. In PS-g-Pole graft copolymers, PS was calculated in the range of 68–87% and Pole was calculated in the range of 12–20%. It can be said that the increase in both polymerization time and polymerization temperature (90 °C) increases PS % and Pole % (Tables 2 and 3).
Investigation of Polymerization Kinetics
It is known that controlled/living polymerization systems occur according to first-order reaction kinetics. The kinetics of the first order reactions are calculated according to the following equation.
where [Mo] and [M] values represent the monomer concentrations at the beginning and at the end of the polymerization reaction at specific times, respectively. The t term is the polymerization time. The term “k”, which is used with concentrations in writing reaction rate relations, is the rate constant of the reaction. The reaction rate constant shows the effect of temperature on the reaction rate and varies from reaction to reaction. Changing the temperature at which the reaction takes place can significantly affect the value of “k”.
In this study, the polymerization was performed at two different temperatures (80 and 90 °C). The effect of temperature on polymerization kinetics was investigated. From the linear parts of the graph ln [Mo]/[M] and t, the rate constant k was calculated for both temperatures and are shown in Figs. 6 and 7, respectively. The rate constant “k” changed significantly with changing the polymerization temperature. Specifically, the rate constants were found to be 1.83 × 10–3 L/mol/dk for the 80 °C reaction and 7.27 × 10–4 L/mol/dk for the 90 °C reaction.
Investigation of Thermal Properties of Graft Copolymers
The thermal properties of the graft copolymers synthesized at both 80 and 90 °C were investigated by thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC). TGA analysis of the graft copolymers revealed two decomposition temperatures. It was observed that the weight loss and decomposition temperatures (Td) of the graft copolymer were at 160 °C corresponding to the Pole and 420 °C corresponding to the PS segments, respectively (Figs. 8 and 9). The thermal transition temperatures of the PS-g-Pole graft copolymers were determined by DSC analysis. A glass transition temperature (Tg) was observed around 60–65 °C for both of the graft copolymers (Fig. 10) with no indication of a melting endotherm. These results suggested that the graft copolymers were either amorphous with limited to no crystallinity or exhibited isodimorphic behavior between the two chain segments of the graft copolymer. Additionally, these results may also be explained by the reduction of homopolymer formation and the high rate of graft copolymer formation.
TGA mass loss curves of PS-g-Pole graft copolymer (PoleM series in Table 3)
dTG curves of PS-g-Pole graft copolymer (PoleM series in Table 3)
Conclusions
In this study, Poly(styrene-graft-oleic acid) graft copolymers with different Pole and PS block lengths were successfully prepared by combining condensation polymerization followed by RAFT polymerization as evidenced by FT-IR, 1H-NMR, GPC, TGA, DSC and SEM–EDX analysis. In general, the molecular weights of the synthesized graft copolymers were higher when produced at 80 °C than those produced at 90 °C at the same polymerization times. However, as expected, increasing polymerization times at each temperature resulted in larger molecular weights until a threshold polymerization time was reached at which the molecular weights stabilized. The rate constant “k” changed significantly with changing the polymerization temperature. Specifically, the rate constants were found to be 1.83 × 10–3 L/mol/dk for the 80 °C reaction and 7.27 × 10–4 L/mol/dk for the 90 °C reaction. A glass transition temperature (Tg) of around 60–65 °C was observed for the graft copolymers synthesized at both 80 and 90 °C and no signs of melting endotherms were observed. These results showed that the graft copolymers were either amorphous and had limited or no crystallinity or exhibited isodimorphic behavior between the two types of chain segments. It can be explained by the good mixing of Pole and PS homopolymers with RAFT polymerization by recording a glass transition temperature (Tg) for graft copolymers. Observation of two different degradation temperatures by TGA analysis may also confirm the formation of bi-block graft copolymer. It can be said that RAFT polymerization at two different temperatures affects the molecular weight of the graft copolymer as well as the polymerization kinetics and accordingly the "k" rate constant. In this contribution, it could be determined that the graft copolymer production by using RAFT and condensation polymerization have superior properties to other traditional polymerization techniques, and examining the kinetic properties of graft copolymers may also contribute to polymer science.
References
Maric M (2021) History of nitroxide mediated polymerization in Canada. Can J Chem Eng 99:832–852. https://doi.org/10.1002/cjce.23989
Matyjaszewski K, Tsarevsky NV (2017) Nanostructured functional materials prepared by atom transfer radical polymerization. Nat Chem 1:276–288. https://doi.org/10.1038/nchem.257
Allı A, Allı S, Becer CR, Hazer B (2016) Nitroxide-mediated copolymerization of styrene and pentafluorostyrene initiated by polymeric linoleic acid. Eur J Lipid Sci Technol 118:279–287. https://doi.org/10.1002/ejlt.201500129
Wang JS, Matyjaszewski K (1995) Controlled/"living" radical polymerization: halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(II) redox process. Macromolecules 28:7901–7910. https://doi.org/10.1021/ma00127a042
Kapil K, Szczepaniak G, Martinez MR, Murata H, Jazani AM, Jeong J, Das SR, Matyjaszewski K (2023) Visible-light-mediated controlled radical branching polymerization in water. Angew Chem Int Ed 62:1–6. https://doi.org/10.1002/anie.202217658
Dworakowska S, Lorandi F, Gorczynski A, Matyjaszewski K (2022) Toward green atom transfer radical polymerization: current status and future challenges. Adv Sci 9:2106076. https://doi.org/10.1002/advs.202106076
Corbin DA, Miyake GM (2022) Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): precision polymer synthesis using organic photoredox catalysis. Chem Rev 122:1830–1874. https://doi.org/10.1021/acs.chemrev.1c00603
Göktaş M, Aykaç C, Öztürk T (2022) One-step synthesis and characterization of the block-graft terpolymer via simultaneous atom transfer radical polymerization (ATRP) and ring-opening polymerization (ROP) techniques. J Chem Sci 134:73. https://doi.org/10.1007/s12039-022-02068-8
Öztürk T, Yavuz M, Göktaş M, Hazer B (2016) One-step synthesis of triarm block copolymers by simultaneous atom transfer radical and ring-opening polymerization. Polym Bull 73:1497–1513. https://doi.org/10.1021/ja00125a035
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH (1998) Living free-radical polymerization by reversible addition—fragmentation chain transfer: the RAFT process. Macromolecules 31:5559–5562. https://doi.org/10.1021/ma9804951
Becer CR, Growth AM, Hoogenboom R, Paulus RM, Schubert US (2008) Protocol for automated kinetic investigation/optimization of the RAFT polymerization of various monomers. QSAR & Comb Sci 27:977–983. https://doi.org/10.1002/qsar.200720159
Aksakal S, Beyer VP, Aksakal R, Becer CR (2019) Copper mediated RDRP of thioacrylates and their combination with acrylates and acrylamides. Polym Chem 10:6622–6629. https://doi.org/10.1039/C9PY01518C
Şanal T, Oruç O, Öztürk T, Hazer B (2015) Synthesis of pH- and thermo-responsive poly(ε-caprolactone-b-4-vinyl benzyl-g-dimethyl amino ethyl methacrylate) brush type graft copolymers via RAFT polymerization. J Polym Res 22:3. https://doi.org/10.1007/s10965-014-0640-z
Whitfield R, Anastasaki A, Nikolaou V, Jones GR, Engelis NG, Discekici EH, Fleischmann C, Willenbacher J, Hawker CJ, Haddleton DM (2017) Universal conditions for the controlled polymerization of acrylates, methacrylates, and styrene via Cu(0)-RDRP. J Am Chem Soc 139:1003–1010. https://doi.org/10.1021/jacs.6b11783
Hazer B, Subramaniyan S, Zhang B (2021) RAFT polymerization of the novel methacrylated methyl salicylate: block copolymerization with poly (3-hydroxy butyrate). ChemistrySelect 6:12255–12265. https://doi.org/10.1002/slct.202102977
Göktaş M (2020) Synthesis and characterization of temperature-responsive block copolymers using macromonomeric initiator. Chem Papers 74:2297–2307. https://doi.org/10.1007/s11696-020-01074-9
Göktaş M, Olgun B (2019) One-step synthesis and characterization of poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) thermo-responsive block copolymers via RAFT and ROP techniques. Polym Sci Ser B 61:421–429. https://doi.org/10.1134/s1560090419040055
Göktaş M, Öztürk T, Atalar MN, Tekeş AT, Hazer B (2014) One-step synthesis of triblock copolymers via simultaneous Reversible-Addition Fragmentation Chain Transfer (RAFT) and ring-opening polymerization using a novel difunctional macro-RAFT agent based on polyethylene glycol. J Macromol Sci Part A-Pure and Appl Chem 51:854–863. https://doi.org/10.1080/10601325.2014.953366
Öztürk T, Atalar MN, Göktaş M, Hazer B (2013) One-step synthesis of block graft copolymers via simultaneous reversible-addition fragmentation chain transfer and ring-opening polymerization using a novel macroinitiator. J Polym Sci Part A Polym Chem 51:2651–2659. https://doi.org/10.1002/pola.26654
Öztürk T, Kayğın O, Göktaş M, Hazer B (2016) Synthesis and characterization of graft copolymers based on polyepichlorohydrin via reversible addition-fragmentation chain transfer polymerization. J Macromol Sci Part A—Pure and Appl Chem 53:362–367. https://doi.org/10.1080/10601325.2016.1166002
Göktaş M, Aykaç C, Akinay Y (2023) Synthesis and characterization of block copolymer: thermal and morphological properties of SiO2-filled block copolymer nanocomposites. Polym Bull 80:8565–8584. https://doi.org/10.1007/s00289-022-04468-9
Frey H, Ishizone T (2017) Living anionic polymerization celebrates 60 years: unique features and polymer architectures. Macromol Chem Phys 218:700217. https://doi.org/10.1002/macp.201700217
Hirao A, Goseki R, Ishizone T (2014) Advances in living anionic polymerization: from functional monomers, polymerization systems, to macromolecular architectures. Macromolecules 47:1883–1905. https://doi.org/10.1021/ma401175m
Larock RC (2010) Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem 12:1893–1909. https://doi.org/10.1039/C0GC00264J
Acikkapi AN, Tuzen M, Hazer B (2019) A newly synthesized graft copolymer for magnetic solid phase microextraction of total selenium and its electrothermal atomic absorption spectrometric determination in food and water samples. Food Chem 284:1–7. https://doi.org/10.1016/j.foodchem.2019.01.091
Kizaloglu A, Kilicay E, Karahaliloglu Z, Hazer B, Denkbas EB (2020) The preparation of chitosan membrane improved with nanoparticles based on unsaturated fatty acid for using in cancer-related infections. J Bioact Compat Polym 35:328–350. https://doi.org/10.1177/0883911520943222
Hazer B (2023) Macro peroxide initiators based on autoxidized unsaturated plant oils: block/graft copolymer conjugates for nanotechnology and biomedical applications. J Am Oil Chem Soc 100:507–520. https://doi.org/10.1002/aocs.12710
Panhwar AH, Tuzen M, Hazer B, Kazi TG (2018) Solid phase microextraction method using a novel polystyrene oleic acid imidazole polymer in micropipette tip of syringe system for speciation and determination of antimony in environmental and food samples. Talanta 184:115–121. https://doi.org/10.1016/j.talanta.2018.03.004
Kocak I, Şanal T, Hazer B (2017) An electrochemical biosensor for direct detection of DNA using polystyrene-g-soya oil-g-imidazole graft copolymer. J Solid State Electrochem 21:1397–1405. https://doi.org/10.1007/s10008-017-3504-8
Allı A, Allı S, Becer CR, Hazer B (2014) One-pot synthesis of poly(linoleic acid)-g-poly(styrene)-g-poly(e-caprolactone) graft copolymers. J Am Oil Chem Soc 91:849–858. https://doi.org/10.1007/s11746-014-2418-1
Yilmazoglu M, Erciyes AT (2018) Styrenation of air blown linseed oil via RAFT–mediated miniemulsion polymerization. Prog Org Coat 114:1–8. https://doi.org/10.1016/j.porgcoat.2017.09.014
Dhakal KH, Jung K-H, Chae JH, Shannon JG, Lee JD (2014) Variation of unsaturated fatty acids in soybean sprout of high oleic acid accessions. Food Chem 164:70–73. https://doi.org/10.1016/j.foodchem.2014.04.113
Xia Y, Larock RC (2010) Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem 12:1893–1909. https://doi.org/10.1039/C0GC00264J
Hazer B, Ayyıldız E, Bahadır F (2017) Synthesis of PNIPAM–PEG double hydrophilic polymers using oleic acid macro peroxide initiator. J Am Oil Chem Soc 94:1141–1151. https://doi.org/10.1007/s11746-017-3020-0
Kim J, Cattoz B, Leung AHM, Parish JD, Becer CR (2022) Enabling reversible addition-fragmentation chain-transfer polymerization for brush copolymers with a poly(2-oxazoline) backbone. Macromolecules 55:4411–4419. https://doi.org/10.1021/acs.macromol.2c00497
Tanaka J, Hakkinen S, Boeck PT, Cong Y, Perrier S, Sheiko SS, You W (2020) Orthogonal cationic and radical RAFT polymerizations to prepare bottlebrush polymers. Angew Chem Int Ed 59:7203–7208. https://doi.org/10.1002/anie.202000700
Alli S, Dulger G, Kiliccioglu AA, Dulger B (2022) Castor oil—based graft copolymers: synthesis, characterization antimicrobial activity and antiproliferative effects against breast cancer cell lines. Polym Bull 79:11177–11199. https://doi.org/10.1007/s00289-021-03908-2
Tuzen M, Elik A, Hazer B, Şimşek S, Altunay N (2020) Poly(styrene)-co-2-vinylpyridine copolymer as a novel solid-phase adsorbent for determination of manganese and zinc in foods and vegetables by FAAS. Food Chem 333:127504. https://doi.org/10.1016/j.foodchem.2020.127504
Rajendran PB, Raghavachari D (2012) Synthesis of graft copolymers onto styrenic polymer backbone via “grafting from” RAFT process. J Polym Sci Part A: Polym Chem 50:4772–4782. https://doi.org/10.1002/pola.26301
Khani MM, Abbas ZM, Benicewicz BC (2017) Well-defined polyisoprene-grafted silica nanoparticles via the RAFT process. J Polym Sci Part A: Polym Chem 55:1493–1501. https://doi.org/10.1002/pola.28514
Bolton J, Tandem JR (2012) RAFT-ATRP synthesis of polystyrene−poly(methyl methacrylate) bottlebrush block copolymers and their self-assembly into cylindrical nanostructures. ACS Macro Lett 1:15–18. https://doi.org/10.1021/mz200003j
Hazer B, Arslan H, Senemoğlu Y (2019) Synthesis of block/graft copolymers based on vinyl benzyl chloride via reversible addition fragmentation chain transfer (RAFT) polymerization using the carboxylic acid functionalized trithiocarbonate. J Polym Res 26:101. https://doi.org/10.1007/s10965-019-1763-z
Göktaş M (2019) Synthesis and characterization of various block copolymers using PMMA-Br macroinitiator. Chem Papers 73:2329–2339. https://doi.org/10.1007/s11696-019-00785-y
Öztürk T, Göktaş M, Hazer B (2010) One-step synthesis of triarm block copolymers via simultaneous reversible-addition fragmentation chain transfer and ring-opening polymerization. J Appl Polym Sci 117:1638–1645. https://doi.org/10.1002/pola.26654
Karahaliloğlu Z, Kilicay E, Hazer B (2022) Herceptin-conjugated magnetic polystyrene-Agsbox nanoparticles as a theranostic agent for breast cancer. J Biomater Appl 36:1599–1616. https://doi.org/10.1177/08853282211065085
Qin A, Sun Z, Zhang Y, Wang Y (2012) Electro-optical properties of polymer dispersed liquid crystal prepared by successively controlled living radical polymerization. Polym Compos 33:178–184. https://doi.org/10.1002/pc.21256
Kilic MS, Korkut S, Hazer B (2017) Novel enzymatic rhodium modified poly(styrene-g-oleic amide) film electrode for hydrogen peroxide detection. Electroanalysis 29:237–2384. https://doi.org/10.1002/elan.201700332
Göktaş M (2019) Synthesis and characterization of poly (styrene-b-methyl methacrylate) block copolymers via ATRP and RAFT. J Inst Sci Tech 9:139–149. https://doi.org/10.21597/jist.435934
Ozturk T, Cakmak I (2008) Synthesis of poly(ethylene glycol-b-styrene) block copolymers by reverse atom transfer radical polymerization. J Polym Res 15:241–247. https://doi.org/10.1007/s10965-007-9164-0
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by the Van Yüzünci Yıl University Scientific Research Fund (Grand Number: FBA-2023-10436) and was supported by the Kapadokya University Research Funds (#KÜN.2020-BAGP-001).
Author information
Authors and Affiliations
Contributions
MG: Supervision, Conceptualization; CA: Conceptualization; BH: Supervision, Conceptualization. RA: Conceptualization.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göktaş, M., Aykaç, C., Hazer, B. et al. Synthesis of Poly(styrene)-g-Poly(oleic acid) Graft Copolymers via Reversible Addition/Fragmentation Transfer (RAFT) Polymerization Using a Poly Oleic Acid Macro-RAFT Agent. J Polym Environ (2024). https://doi.org/10.1007/s10924-023-03144-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-023-03144-x