Abstract
Replacing conventional plastics with other products obtained from biobased and biodegradable raw materials is an increasingly studied solution. In this line, the development of protein-based bioplastics is a promising alternative. However, for some applications, such as packaging it would be necessary to improve their properties by including an additional stage to crosslink the protein chains during the production of bioplastics. Therefore, pea protein-based bioplastics crosslinked with genipin, a natural non-toxic chemical crosslinking agent, are evaluated in this study. The bioplastics are obtained via injection moulding and genipin, is included in the initial formulation. Specifically, the concentration of genipin and the effects on the protein blends with genipin over days are evaluated. The evolution of the reaction is followed by the colour change of the blend, together with thermal analyses and infrared spectroscopy. Results showed the evolution of the crosslinking in the blends resulted in conformational changes that resulted in the modification of the initial yellowish colour to a blueish system. Respect their bioplastics, more deformable systems with a lower water absorption capability are obtained by using genipin as crosslinking agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industrial plastic production began in 1950, and since then, approximately 8.3 billion tons of plastic materials have been produced [1]. However, there are significant drawbacks derived from the high generation of plastic wastes after their use. There are different ways of plastic disposal that have been traditionally used such as incineration, landfills or recycling. Recycling is the most popular disposal treatment since it presents some advantages such as the generation of less CO2 emissions than other traditional alternatives [2], together with the possibility of reusing already used plastic. It is pursued in order to stop using non-renewable resources.
Another of the many contributions that must be made to achieve fully sustainable development is the development of new materials that can replace conventional plastics. These materials are obtained from biobased and biodegradable resources, avoiding many of the drawbacks of conventional plastics, such as their low degradability or the generation of toxic products [3, 4]. Following this strategy, bioplastics obtained from proteins extracted from residues of the agri-food industry have been developed in recent years [5]. However, the main inconvenience related to the fabrication of new bioplastics as potential substitutes for conventional plastics are their deficient properties as their low mechanical and barrier properties, the high price of obtaining the raw material, or the lack of industrial waste management systems that can adequately handle bioplastics, despite their good biodegradability. In this sense, more research on these issues is necessary for the correct implementation of these materials in the industrial sector.
Modifying the properties of protein-based materials with the formation of new bonds is of great significance to suppy the requeriments for applications in many fields such as food packaging, tissue engineering and biomedical research [6, 7]. Sometimes some additional crosslinking stages are included to form new bond that induce changes in the structure of protein-based bioplastics. Thus, this crosslinking stage could modify the microstructural properties of the materials as well as their mechanical and functional properties [8, 9].
The crosslinking stage may be carried out with either a physical or chemical crosslinking. Physical crosslinking is associated to non-covalent secondary interactions between polymer chains such as electrostatic interactions, hydrogen bonds and hydrophobic forces [10]. Its use prevents the addition of external compounds that may grant toxicity to the material, although the properties of the products could be improved. In this sense, the use of chemical crosslinking causes a greater impact on the characteristics of the resulting materials [11].
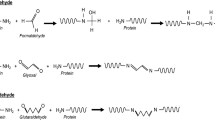
To curb the use of crosslinking agents with a marked toxic character, such as glutaraldehyde or formaldehyde, more and more alternatives are emerging [12]. In this sense, genipin has been recently investigated as a green crosslinking agent [13]. The remarkable characteristic of genipin is its ability to react with primary amines, giving rise to chemically crosslinked structures, which allows its use in drug delivery systems such as microcapsules and hydrogels. Among the most studied there are chitosan hydrogels crosslinked with genipin, such as the study of Fwu-Long et al., in which chitosan microspheres are prepared by a water-in-oil dispersion method, using genipin as a crosslinking agent [14]. Furthermore, Yuan et al. obtained complex systems using chitosan, bovine albumin and genipin to form microspheres [15].
Genipin is a compound of natural origin that is obtained from the geniposide from the fruit of Gardenia jasminoides and from the fruit of Genipa americana. The still green fruits provide a blue colour, which is used for various purposes [16]. The coloring power is due to the presence of an iridoid called genipin, which is initially colorless but turns blue in contact with amino groups [17]. Furthermore, in addition to its crosslinking capacity, genipin has pharmacological properties such as anti-inflammatory, diuretic, choleretic and hemostatic [18, 19].
The main advantage of using genipin as a potential crosslinking agent lies in its low toxicity. In this sense, the main novelty of this work is related to the development of pea protein-based bioplastics with optimized properties by genipin crosslinking. This evaluation is carried out following the evolution of the crosslinking of pea protein-based systems crosslinked with genipin over time. Therefore, the morphological, mechanical and functional properties of the bioplastics were measured and compared over the curing days of the blends with different genipin content in the formulation.
Materials and Methods
Materials
Bioplastics were based on pea protein isolate (PPI, ca. 90 wt% of protein), which was purshased by Roquette (France). Genipin (≥ 98% (HPLC), powder) was supplied by Guangxi Shanyun Biochemical Science and Technology Co., Ltd. (China). Furthermore, glycerol, which was provided by Panreac Química S.A. (Spain), was used as plasticizer.
System Preparation
Bioplastic Production
Pea protein-based bioplastics were obtained by means of an injection moulding process, which comprises two stages: first, a mixing stage at 25 ºC was performed in a HaakePolylab QC batch mixer (ThermoHaake, Germany) to produce a homogeneous blend. The blends were prepared with a 1.5/1 protein/glycerol ratio and a 0.25 and 0.50 wt% of genipin [20]. The addition of glycerol as plasticizer is essential to promote the processability of the resulting blend [21]. Secondly, once the blends were homogenized, they were injected in a MiniJet Piston Injection Moulding System (ThermoHaake). The processing conditions were: 50 and 130 °C (as cylinder and mould temperatures) and 500 bar for 20 s and 200 bar during 200 s (as injection and post-injection conditions). Two different specimens were fabricated: 60 × 10 × 1-mm3 rectangular-shaped specimen and dumbbell-shaped specimen (type V) according to ISO 527-1:2012.
Crosslinking of Bioplastics
Different concentrations of genipin were used (0.25 and 0.50%) for the crosslinking of pea protein-based bioplastics, evaluating the curing time of the blend after the mixing stage and performing the injection stage at different times (day 0, 1, 5 and 10). Tables 1 and 2 shows a summary of the different samples carried out.
Characterization Techniques
Characterization of Blends
Colour Measurements
Colour analysis is performed using a KONICA MINOLTA CM-700D spectrocolorimeter (Japan). The value of L* (lightness) and a and b (red, green, blue and yellow in CIELAB colour space) was determined for each system as the average of five scans. The parameter a drifts from negative values (green) to positive values (red), whereas b drifts from negative to positive values when the sample is blue or yellow, respectively.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra in transmission mode of both pure components (pea protein and genipin) and the resulting pea protein-based blends were acquired over a wavenumber range from 400 to 4000 cm−1 at 4 cm−1 resolution using a FT/IR-4200 spectrometer (JASCO, Tokyo, Japan). KBr was used as the medium to prepare the different samples (in a concentration of 1/10 of the solid sample in a microspatula), which were placed into a holder in the FTIR device. The evolution of the peaks from Amide I, II and III signals has been analyzed following the peak area changes (P2) with respect a reference peak area (P1) as shown in Eq. (1):
Differential Scanning Calorimetry (DSC)
10 to 20 mg of different blends and the pure materials were sealed in aluminum pans and placed in the Q250 DSC calorimeter (TA Instruments New Castle, USA). Tests were carried out under N2 atmosphere at a flow rate of 50 ml min−1 while applying a temperature program by heating from 30 to 280 °C at 10 °C min−1.
Thermogravimetric Analysis (TGA)
The loss of mass with a temperature increment of the different pea protein-based blends was evaluated by means of TGA tests, using a thermal analyser Q-50 (TA Instruments Water, USA). For this, 10–20 mg of sample was placed into a platinum pan and subjected a temperature ramp from 30 to 600 °C under nitrogen atmosphere. The temperature was increased 10 °C min− 1.
Temperature Ramps
The behaviour of the blends with temperature was analyzed by carrying out temperature ramps in the range 25–150 ºC in compression mode (at constant strain and 1 Hz) using a DMA850 Discovery (TA Instruments, USA). The temperature was increased 10 °C min−1.
Characterization of Bioplastics
Crosslinking degree
The protocol described by Zárate et al. was used to determine the crosslinking degree of the different samples [22]. Briefly, 170–180 mg of bioplastic was denatured with 5 mL of sodium dodecyl sulphate (SDS) solution which solubilizes the uncrosslinked protein. Then, the Lowry’s method was used to determine the solubilized protein [23]. The crosslinking degree was calculated respect to a reference system. In this way, pea protein bioplastic without any crosslinking stage was selected as reference.
Dynamic Mechanical Analysis (DMA)
DMA tests were performed with a RSA3 analyser (TA Instruments, USA) with a three-point bending geometry in a flexural mode. For this, rectangular probes were used. Firstly, strain sweep tests (0.002–1% of strain at a constant frequency of 1 Hz and 20–22 ºC) were performed to obtain the linear viscoelastic range. In addition, frequency sweep tests were carried out between 0.02 and 20 Hz within the linear viscoelastic range at 20–22 ºC.
Tensile Strength Measurements
Tensile tests until break were performed in a universal machine Insight 10 kN Electromechanical Testing System (MTS, USA). Samples were subjected to an increased uniaxial force at 10 mm/min and room temperature. The ISO 527-2 standard for Tensile Properties of Plastics was followed [24]. The parameters obtained were: the Young’s modulus (E), the maximum tensile strength (σmax) and the strain at break (εmax).
Water Uptake Capacity and Soluble Matter Loss
The ability to absorb water by the bioplastic was determined with the ASTM D570 standard [25]. For this, rectangular bioplastics were firstly dried in an oven at 50 ºC for 1 h to obtain the initial dry weight. Later, they were immersed in 300 mL of distilled water for 24 h in a closed vessel (wet weight). Finally, the bioplastics were dried again in the oven to obtain the final dry weight. Water uptake capacity (WUC) and soluble matter loss were determined by Eqs. (2) and (3):
Statistical Analysis
Each measurement was presented as the mean of at least three replicates with their standard deviation (mean ± SD). In addition, statistical analyses were carried out throughout t-test and one-way analysis of variance (ANOVA), selection a confidence level of 95% (p < 0.05). These analyses were performed in the statistical package Excel 2013 (Microsoft, Redmond, WA, USA). Standard deviations from some selected parameters were calculated.
Results and Discussion
The chemical crosslinking of pea protein was carried out using genipin. Genipin is a crosslinking agent which reacts with primary amines, giving rise to chemically crosslinked structures. In this sense, according to the amino acid profile of pea protein, presenting a high content of Arginine and Lysine, which means that the crosslinking with genipin may be effective (see Fig. 1). On the other hand, as shown by Makita et al., the use of genipin, in addition to generating crosslinking, produces a blueish colour in the materials in which it is used [26]. The reaction is mainly based on the nucleophilic attack of a primary amine on genipin, causing the opening of the dihydropyran ring, then the resulting secondary amine attacks the aldehyde group generated [27]. This reaction is promoted by the presence of oxygen and expedited when temperature increases [28].
Blends
Colour Measurements
The aspect of the pea protein blend with genipin in its formulation was recorded over days to notice any possible changes occurred. The macroscopic aspect of the mixed blends obtained at different curing times is shown in Fig. 2. There is a modification of the blend colour towards a green/blueish colour, which according to Makita et al. is related to an evolution in the crosslinking of the system produced by the interaction of genipin with the free primary amines present in the protein chains [26]. A colour change is appreciable for both blends crosslinked with 0.25 and 0.50% genipin. Carrying out the crosslinking process with a 0.25% of genipin, the colour change is more noticeable from the system 25D5 onwards, obtaining a bluish green hue. As some authors reported, the intermediate groups produced during the reaction could give rise to this colour modification thanks to the nucleophilic attack of methylamine on the olefinic carbon atom at C3 of deoxyloganin aglycone, together with the opening of the dihydropyran ring and attacked by the secondary amino group on the resulting aldehyde group, which generates the formation of genipin-methylamine monomers [27]. On the other hand, 0.50% genipin blends presented a more bluish colour from 50D1 up to 50D10. Interestingly, both 25D0 and 50D0 showed a similar aspect and colour to the non-crosslinked blend (PP). Respect the non-crosslinked reference blend (PP); it did not undergo any change over time, unlike the blends with genipin.
Images of the pea protein blends crosslinked by the addition of genipin at 0.25 and 0.50% at different curing times of the blend (Day 0, Day 1, Day 5 and Day 10): B 25D0, C 25D1, D 25D5, E 25D10, B’ 50D0, C’ 50D1, D’ 50D5 and E’ 50D10. Images of the pea protein flour (A) and the reference blend (PP) without any crosslinking agent A’ are also included
The values of the colour parameters obtained for the pea protein flour and the blends with and without genipin are shown in Tables 1 and 2. The reference blend (PP) and the protein flour presented a yellowish colour as shown by the high positive values of all the parameters (a, b and L*). Regarding the blends of the systems with genipin, evolution can be observed with the curing time. The systems 25D0 and 25D1 showed values similar to those obtained by the PP system. However, from 25D5 onwards, there was a change in coloration towards a bluish green hue caused by the genipin present (Fig. 2). This change is noticeable for the decrease in parameters a and b (especially in parameter a as the green-like colour of the resulting blend). On the other hand, the system processed with 0.50% genipin showed similar values at 50D0 as PP blend. However, the effect of genipin became more significant from 50D1, producing a significant decrease in all the parameters, not showing significant changes until 50D10, as can be seen in Fig. 2; Tables 1 and 2.
FTIR Tests
The chemical crosslinking of pea protein-based blends with genipin was followed by FTIR analysis. Figure 3 shows the infrared spectra for all blends in comparison with the raw materials (pea protein and genipin) as well as the non-crosslinked reference system (PP) as a function of the curing time (Fig. 2A and B). As can be appreciated, FTIR measurements of several crosslinked systems were monitored (25D0, 25D1, 50D0 and 50D1), and several differences in their fundamental bands could be detected as the chemical reaction took place, especially in the wavenumber range of 2000–1000 cm−1. In previous works, some authors have reported the infrared spectroscopy curves of pea protein [29], identifying the most relevant peaks at 3000–3500 cm−1 (tensile vibration of OH and NH2 groups), 2933 cm− 1 (–CH stretching vibration), 1600–1700 cm−1 (amide I, C=O and C–N stretching vibration and the backbone conformation of proteins), 1500–1600 cm−1 (amide II, N–H and C–N vibration bands) and at 1200 cm−1 (amide III, C–N stretching and N–H deformation) [30]. On the other hand, the crosslinking agent (genipin molecule) showed two significant vibration bands at around 1680 and 1620 cm−1, related to stretching vibration of carboxymethyl group (C=O) and C=C vibration of olefin ring in genipin, respectively [31]. In this sense, the chemical reaction occurring between genipin and pea protein (see Fig. 1) and the subsequent crosslinking can be deduced from the FTIR curves shown in Fig. 3A. The chemical interaction comprising NH2 groups of pea protein lysine molecule and genipin molecular structure (see the chemical reaction in Fig. 1) could be corroborated thanks to an increase in the vibration bands related to amide I, II and III regions (1650, 1550 and 1200 cm−1, respectively). This noticeable magnification confirms the presence of a higher number of C=O, C=C and N–C groups in the chemical structure of the final blend due to both the introduction of genipin molecule and the formation of aromatic amine groups as a consequence of chemical crosslinking [31]. Thus, by calculating the peak areas of these main vibration bands and considering the glycerol absorption peak (1040 cm−1, which remains constant during the chemical reaction) as a reference vibration absorption signal [30], the progress of the crosslinking reaction can be followed by FTIR techniques. The evolution of these areas (P2) can be seen in Fig. 3B and D, where the values of such peak areas [amide I (1650 cm−1), amide II (1550 cm−1) and amide III (1200 cm−1) regions] have been plotted as a function of the reference vibration band area (P1) versus time. As can be deduced from the curves, the highest increase in such area values was obtained for the band related to amide III (C–N bonds), which also became broader for the crosslinked blends (those containing genipin). It was related to a higher amount of amide bonds comprising the formulations as a consequence of the formation of C–N bonds during the development of heterocyclic genipin crosslinked compounds [32]. On the other hand, it should be noticed that although the curing process was monitored by FTIR tests only for D0 (25D0 and 50D0) and D1 (25D1 and 50D1) systems, a higher rate in the curing process could be detected when increasing the amount of genipin used during the reaction process, as deduced from the slopes plotted in Fig. 3B and D. The system 50D0 showed the highest peak area when crosslinking with 0.50% of genipin, whilst for 0.25% of genipin, the 25D1 system was the one that achieved the highest peak area in the Amide III region. These results agree with the images observed for the colour analysis, with a faster and more marked change in the systems with a higher concentration of genipin.
Infrared spectroscopy of pea protein blends crosslinked A with 0.25% genipin at different curing times of the blend: Day 0 (25D0) and Day 1 (25D1); and bioplastics crosslinked (C) with 0.50% genipin at different curing times of the blend: Day 0 (50D0) and Day 1 (50D1). Evolution of the Amide I, II and III peaks over time were also plotted for blends crosslinked B with 0.25% genipin and D with 0.50% genipin at different curing times. Non-crosslinked pea protein blend (PP) and pea protein and genipin powders were also included
TGA
In addition, the chemical crosslinking reaction was also monitored by thermogravimetric analysis. Figure 4 shows a comparative representation of the loss of weight as the derived weight of the raw materials (pea protein flour and genipin powder) and their blends with different genipin amounts as a function of the curing time (25D0, 25D1, 50D0 and 50D1). The thermal decomposition of pea protein displayed an initial loss of weight due to humidity loss at a temperature lower than 100 ºC. Besides, a higher decomposition event appeared when raising temperature more than 200 ºC, from which the polysaccharide structure started to decompose by means of C–O–C and C–C bonds rupture, also including the volatilization of other compounds [33].
On the other hand, genipin showed a single thermal peak between 180 and 280 ºC (Fig. 4B), where the degradation of genipin structural network took place by means of C=C and CO breakdown together with molecule dehydration [34]. In this sense, some differences as a function of curing time can be noticed in the graph for crosslinked blends, mostly in the temperature range from 100 to 250 ºC, which shifted this degradation peak as the curing time increased. Thus, system 50D0 showed two overlapped peaks in this temperature range (⁓200 and 240 ºC), due to the existence of a non-homogeneous system and the subsequent thermal degradation of both mixture matrix and non-crosslinked genipin, respectively. However, as curing time increased and the crosslinking reaction progressed (50D1), a thermal degradation profile similar to those shown by the reference system (PP) was obtained as a result of the introduction of the total genipin amount into the polymer chemical microstructural network. This fact also corroborates the crosslinked network formed between raw materials. However, this modification in the microstructure caused by the genipin does not generate significant effects in the TGA with respect to the reference system.
A Thermogravimetric analysis and B derivative weight of pea protein blends crosslinked with 0.25% genipin at different curing times of the blend: Day 0 (25D0) and Day 1 (25D1); and C Thermogravimetric analysis and D derivative weight of pea protein blends crosslinked with 0.50% genipin at different curing times of the blend: Day 0 (50D0) and Day 1 (50D1). Non-crosslinked pea protein blend (PP) and pea protein and genipin powders were also included
DSC
Differential scanning calorimetry (DSC) curves relative to the first heating of samples and providing information about the thermal properties of biopolymers and their evolution with curing time are shown in Fig. 5. Protein thermal behaviour showed properties of a semicrystalline polymer, in which two endothermic thermal events could be detected in the range of 30–250 ºC. Thus, as can be seen from Table 3, a glass transition at around 156 ºC together with a melting transition (171 ºC, 155 J/g) were identified in pea protein curves. These temperatures were shifted when including glycerol into the formulation (PP), being 150 and 177 ºC (127 J/g), respectively [30].
On the other hand, some differences could also be detected in the thermal events when genipin is added as a crosslinking agent. In such a way, these blends comprise numerous interactions involving polysaccharides and amino groups, which result in an increase in intermolecular bonding, and lead to a reduction in chain mobility with a subsequent increase in Tg (from 150 to 157 ºC) [35, 36]. Moreover, as can be seen in Fig. 5, a displacement of both thermal events, Tg and melting point (Tm), was detected as the crosslinking reaction moved forward due to the higher number of inter- (and intra-) molecular associations with the amino groups of pea protein. Furthermore, it should be noticed that crosslinking generally induces a reduction in melting enthalpy (from 304 to 169 J/g for 25D0 and 25D1 systems and 285 to 129 J/g for 50D0 and 50D1 systems, respectively) because of both a decrease in the hydrogen bonds forming the microstructural network, which breaks endothermically and a simultaneous increase in the extent of covalent crosslinks, which exothermic ruptures [37].
Differential scanning calorimetry of blends crosslinked A with 0.25% genipin at different curing times of the blend: Day 0 (25D0) and Day 1 (25D1); and bioplastics crosslinked B with 0.50% genipin at different curing times of the blend: Day 0 (50D0) and Day 1 (50D1). Non-crosslinked pea protein blend (PP) and pea protein and genipin powders were also included
Temperature Ramps
Temperature ramps have also been carried out for the blends processed with 0.25% (Fig. 6A) and 0.50% genipin (Fig. 6B). The reference system without any crosslinking agent (PP) showed a decrease in the E’ Different letters were included as superscripts to denote significant differences between the values values until an inflection point. Then, there is an increase in the E′ values probably because the low molecular weight of pea protein [38] facilitates the interaction between the biopolymeric chains and, consequently, resulting in the strengthening of the bioplastic [21].
Temperature ramps of bioplastics crosslinked (A) with 0.25% genipin at different curing times of the blend: Day 0 (25D0B), Day 1 (25D1B), Day 5 (25D5B), Day 10 (25D10B); and bioplastics crosslinked (B) with 0.50% genipin at different curing times of the blend: Day 0 (50D0B), Day 1 (50D1B), Day 5 (50D5B), Day 10 (50D10B). Non-crosslinked pea protein-based bioplastic (PPB) was also included as reference
The strengthening observed at 125 ºC for the PPB system is also observed by the 25D0 and 25D1, probably because in this system, the interaction with genipin occurs slower and the effect of temperature takes place. However, for 25D5 and 25D10 systems, greater interaction with genipin took place and, as a consequence, the hardening of the material and the E′ increase is not observed with temperature. A similar behavior took place with the bioplastics obtained with 0.50% genipin (Fig. 6B). The increase in the E′ values after the minimum occurs for the 50D0 system. However, from 50D1 the values of E′ reached a plateau region where this hardening is not observed since having genipin at a higher concentration, the interaction took place earlier, so that rise with temperature did not occur. So, the curing process occurs faster when the concentration of genipin was higher, as observed previously in the colour change of the blends and corroborated by the FTIR and TGA analyses.
According to the results obtained, the conformational change induced by the presence of genipin influences the thermal properties of the resulting material, since the thermal strengthening appeared at lower temperatures than the PPB system and it did not occur when the curing time increased for both 0.25 and 0.50% genipin-crosslinked bioplastics. Furthermore, there is a softening of the elastic moduli when the systems are crosslinked, as previously observed by Jiménez-Rosado et al. [4].
Bioplastics
Physicochemical Properties
The crosslinking degree of the pea protein-based bioplastics crosslinked with genipin at 0.25 and 0.50% is summarized in Table 4. As it can be seen, evaluating the concentration of genipin for the whole curing time studied, the higher the concentration, the higher the crosslinking degree of the systems. Bioplastics obtained with a 0.25% showed a crosslinking degree up to 11% with respect to the reference system, whereas 0.50% genipin obtained a crosslinking degree of ca. 23%.
On the other hand, the colour parameters (a, b and L*) for the different systems are shown in Table 5. As can be seen, the crosslinked systems with genipin show a significant decrease in the values of parameters a and b due to a change in the colour of the bioplastics from an orange-yellow tone to a dark blue colour. In this sense, a darkening of the sample is also observed as a consequence of the decrease observed in the parameter L* with respect to the reference. This colour change towards a blue hue, caused by the addition of genipin, has been observed by other authors in previous studies [39]. Comparing the colour of the bioplastics and the blends (Table 5), the injection moulding process promoted the reaction between pea protein and genipin since there was a change in the colour for all the systems studied. In other words, bluish bioplastics were obtained independently of the colour of the blend used.
Mechanical Properties
The profiles obtained for the frequency sweep tests can be seen in Fig. 7. Considering the bioplastics crosslinked with 0.25% genipin (Fig. 7A), an increase in the curing time of the blend led to an increase in the instability of the bioplastics at low frequencies, especially from 25D5B onwards. However, 0.50% crosslinked bioplastics only present a slight slope (Fig. 7B) that generates an increase in E’ values at higher frequencies, probably because the crosslinking reaction occurred faster, stabilizing the system earlier.
Flexural frequency tests of bioplastics crosslinked A with 0.25% genipin at different curing times of the blend: Day 0 (25D0B), Day 1 (25D1B), Day 5 (25D5B), Day 10 (25D10B); and bioplastics crosslinked B with 0.50% genipin at different curing times of the blend: Day 0 (50D0B), Day 1 (50D1B), Day 5 (50D5B), Day 10 (50D10B). Non-crosslinked pea protein-based bioplastic (PPB) was also included as reference
In general, there is a reinforcement of the crosslinked structures as seen by the higher E′ values regarding the PPB bioplastic, which is more significant for the 0.50% bioplastics. Comparing E′ values at 1 Hz (E′1) shown in Tables 6, it is observed that the systems present an elastic modulus (E′ values) lower as the curing time increases, although without significant differences for 50D5B and 50D10B. Genipin crosslinked bioplastics presented higher E′ values than other studied bioplastics obtained with rice or soy protein [40, 41]. In this sense, these results correlate with previous studies since some authors obtained enhanced mechanical strength of protein-based materials with the addition of genipin as crosslinking agent [42, 43].
Figure 8 shows the tensile profiles obtained for the systems with a 0.25% (Fig. 8A) and 0.50% (Fig. 8B). As it can be seen, all the systems exhibited a similar profile. A first elastic region that corresponds with a linear profile, followed by a continuous decrease in the stress-strain slope (plastic region). Finally, each system broke down at a different elongation after passed through a maximum stress value.
Tensile tests of bioplastics crosslinked A with 0.25% genipin at different curing times of the blend: Day 0 (25D0B), Day 1 (25D1B), Day 5 (25D5B), Day 10 (25D10B); and bioplastics crosslinked B with 0.50% genipin at different curing times of the blend: Day 0 (50D0B), Day 1 (50D1B), Day 5 (50D5B), Day 10 (50D10B). Non-crosslinked pea protein-based bioplastic (PPB) was also included as reference
Table 6 shows the tensile parameters obtained from the profiles in Fig. 8. Regarding Young’s modulus and maximum stress, the same effect is observed, a decreasing trend occurred when increasing the genipin concentration. Furthermore, there was a decrease in Young’s modulus with the curing time, being also lower than the PPB (reference) system without any crosslinking agent. This effect is more significant for the 0.50% genipin-based bioplastics. On the other hand, the strain at break exhibits a slightly increase with the addition of genipin with respect to the reference system. This effect is similar to the results obtained by previous studies, in which the addition of genipin decreased the rigidity but improved the deformability of the final materials [19, 44].
Water Uptake Capacity
Water uptake values of the different systems with and without any crosslinking agents are included in Fig. 9.
Water uptake capacity and soluble matter loss of bioplastics crosslinked with 0.25% genipin at different curing times of the blend: Day 0 (25D0B), Day 1 (25D1B), Day 5 (25D5B), Day 10 (25D10B); and bioplastics crosslinked with 0.50% genipin at different curing times of the blend: Day 0 (50D0B), Day 1 (50D1B), Day 5 (50D5B), Day 10 (50D10B). Non-crosslinked pea protein-based bioplastic (PPB) was also included as reference
It can be seen how the addition of genipin produces a decrease in the water uptake capacity of the bioplastics, being for all the systems lower than the reference system. Comparing the different systems, the curing time did not affect the absorption uptake since there are no significant differences between the systems obtained at 0.25% or for those obtained at 0.50% concentration of genipin. Regarding the evaluation of the concentration of the crosslinking agent, it was demonstrated how, by increasing the concentration of the crosslinker, there is a decrease in the water uptake capacity of the obtained bioplastics. This decrease may be due to a combination of two effects. On the one hand, the addition of genipin generates a change in the microstructure that gives the material a greater hydrophobic character due to the presence of aromatic groups in the final structure, together with the disruption of the hydrogen bond forming as observed by the DSC tests. Similar results were obtained by other authors, with a decrease in the wettability of protein-based systems with the addition of genipin [45]. On the other hand, the higher crosslinking degree of the systems may induce lower mobility of the protein chains and, therefore, decrease the water absorption capability. Nevertheless, the addition of genipin as the crosslinking agent did not influence the soluble matter loss, since for all systems is in the range of 45–50%. The decrease in the water absorption properties of crosslinked protein-based bioplastics was also observed in previous studies [46].
Conclusions
Pea protein-based bioplastics with tunable properties have been processed by adding genipin as crosslinking agent during the thermomoulding fabrication process.
The evaluation of the blends revealed that the crosslinking reaction can be followed by infrared spectroscopy or thermal analyses, as well as by the colour change towards a blue hue, macroscopically observed. FTIR results matched DSC and TGA tests concluding that a higher rate of the curing process took place by increasing the concentration of genipin used. The properties of these bends make them candidates for the formation of bioplastics with tuned characteristics.
Respect the bioplastics, a higher crosslinked degree was found by increasing the concentration of genipin in the initial formulation. Furthermore, the addition of genipin to the initial formulation produced more deformable bioplastics with lower absorption capability due to the conformational changes observed in the protein changes. This work opens the potential of these genipin-crosslinked bioplastics for their use in applications where greater manageability of the materials is necessary (less hard and more flexible materials) and that have a certain capacity to absorb water, such as medicine (i.e. bandages), pharmacy or agriculture (i.e. controlled release systems of drugs or nutrients).
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
European Bioplastics (2019) Bioplastics market data 2019. Global production capacities of bioplastic 2019–2024. Eur Bioplastic 9:1–14
Van Roijen EC, Miller SA (2022) A review of bioplastics at end-of-life: linking experimental biodegradation studies and life cycle impact assessments. Resour Conserv Recycl 181:106236. https://doi.org/10.1016/j.resconrec.2022.106236
Michael AH (2017) Horticulture plastics. Elsevier Ltd
Jiménez-Rosado M, Rubio-Valle JF, Perez-Puyana V et al (2020) Eco-friendly protein-based materials for a sustainable fertilization in horticulture. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.124948
Jiménez-Rosado M, Zarate-Ramírez LSS, Romero A et al (2019) Bioplastics based on wheat gluten processed by extrusion. J Clean Prod 239:117994. https://doi.org/10.1016/j.jclepro.2019.117994
Gómez-Heincke D, Martínez I, Partal P et al (2016) Development of antimicrobial active packaging materials based on gluten proteins. J Sci Food Agric 96:3432–3438. https://doi.org/10.1002/jsfa.7525
Jones A, Mandal A, Sharma S (2015) Protein-based bioplastics and their antibacterial potential. J Appl Polym Sci 132:41931. https://doi.org/10.1002/app.41931
Jiménez-Rosado M, Bouroudian E, Perez-Puyana V et al (2020) Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.121517
Awadhiya A, Kumar D, Verma V (2016) Crosslinking of agarose bioplastic using citric acid. Carbohydr Polym 151:60–67. https://doi.org/10.1016/j.carbpol.2016.05.040
Yu AC, Lian H, Kong X et al (2021) Physical networks from entropy-driven non-covalent interactions. Nat Commun 12:746. https://doi.org/10.1038/s41467-021-21024-7
Oryan A, Kamali A, Moshiri A et al (2018) Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol 107:678–688. https://doi.org/10.1016/j.ijbiomac.2017.08.184
Khan A, Gallah H, Riedl B et al (2016) Genipin cross-linked antimicrobial nanocomposite films and gamma irradiation to prevent the surface growth of bacteria in fresh meats. Innov Food Sci Emerg Technol 35:96–102. https://doi.org/10.1016/j.ifset.2016.03.011
Yoo JS, Kim YJ, Kim SH, Choi SH (2011) Study on genipin: a new alternative natural crosslinking agent for fixing heterograft tissue. Korean J Thorac Cardiovasc Surg 44:197–207. https://doi.org/10.5090/kjtcs.2011.44.3.197
Mi F-L, Sung H-W, Shyu S-S (2001) Release of indomethacin from a novel chitosan microsphere prepared by a naturally occurring crosslinker: examination of crosslinking and polycation-anionic drug interaction. J Appl Polym Sci 81:1700–1711. https://doi.org/10.1002/app.1602
Yuan Y, Chesnutt BM, Utturkar G et al (2007) The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydr Polym 68:561–567. https://doi.org/10.1016/j.carbpol.2006.10.023
Brauch JE (2016) Underutilized fruits and vegetables as potential novel pigment sources. Handbook on natural pigments in Food and Beverages. Elsevier, pp 305–335
Pal K, Paulson AT, Rousseau D (2009) Biopolymers in controlled-release Delivery Systems. In: Modern Biopolymer Science, Elsevier, pp 519–557
Panchal R, Mateti T, Likhith K et al (2022) Genipin cross-linked chitosan–PVA composite films: an investigation on the impact of cross-linking on accelerating wound healing. React Funct Polym 178:105339. https://doi.org/10.1016/j.reactfunctpolym.2022.105339
Perez-Puyana V, Rubio-Valle JF, Jiménez-Rosado M et al (2020) Chitosan as a potential alternative to collagen for the development of genipin-crosslinked scaffolds. React Funct Polym 146:104414. https://doi.org/10.1016/j.reactfunctpolym.2019.104414
Dimida S, Barca A, Cancelli N et al (2017) Effects of Genipin Concentration on Cross-Linked Chitosan Scaffolds for bone tissue Engineering: structural characterization and evidence of Biocompatibility features. Int J Polym Sci 2017:1–8. https://doi.org/10.1155/2017/8410750
Jiménez-Rosado M, Rubio‐Valle JF, Perez‐Puyana V et al (2021) Comparison between pea and soy protein‐based bioplastics obtained by injection molding. J Appl Polym Sci 138:50412. https://doi.org/10.1002/app.50412
Zárate-Ramírez LS, Romero A, Martínez I et al (2014) Effect of aldehydes on thermomechanical properties of gluten-based bioplastics. Food Bioprod Process 92:20–29. https://doi.org/10.1016/j.fbp.2013.07.007
Markwell MAK, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87:206–210. https://doi.org/10.1016/0003-2697(78)90586-9
ISO 527-2:2012. Plastics—Determination of tensile properties-Part 2: Test Conditions for Moulding and Extrusion Plastics. pp 527–532
ASTM (American Society for Testing and materials) “Standard test method for plastic”, Annual book of ASTM standards Philadelphia. PA: American Society for Testing and Materials
Makita R, Akasaka T, Tamagawa S et al (2018) Preparation of micro/nanopatterned gelatins crosslinked with genipin for biocompatible dental implants. Beilstein J Nanotechnol 9:1735–1754. https://doi.org/10.3762/bjnano.9.165
Touyama R, Inoue K, Takeda Y et al (1994) Studies on the Blue Pigments Produced from Genipin and Methylamine. II.On the formation mechanisms of brownish-red Intermediates leading to the blue pigment formation. Chem Pharm Bull (Tokyo) 42:1571–1578
Pizzolitto C, Cok M, Asaro F et al (2020) On the mechanism of Genipin binding to primary Amines in Lactose-Modified Chitosan at Neutral pH. Int J Mol Sci 21:6831. https://doi.org/10.3390/ijms21186831
Türker-Kaya S, Huck C (2017) A review of Mid-Infrared and Near-Infrared imaging: principles, concepts and applications in Plant tissue analysis. Molecules 22:168. https://doi.org/10.3390/molecules22010168
Acquah C, Zhang Y, Dubé MA, Udenigwe CC (2020) Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr Res Food Sci 2:61–69. https://doi.org/10.1016/J.CRFS.2019.11.008
Kahoush M, Behary N, Guan J et al (2021) Genipin-mediated immobilization of glucose oxidase enzyme on carbon felt for use as heterogeneous catalyst in sustainable wastewater treatment. J Environ Chem Eng 9:0–2. https://doi.org/10.1016/j.jece.2021.105633
Liu J, Liu CK, Brown EM (2017) Development and characterization of Genipin cross-linked gelatin based Composites Incorporated with Vegetable-Tanned collagen Fiber (VCF). J Am Leather Chem Assoc 112:410–419
Ricci L, Umiltà E, Righetti MC et al (2018) On the thermal behavior of protein isolated from different legumes investigated by DSC and TGA. J Sci Food Agric 98:5368–5377. https://doi.org/10.1002/jsfa.9078
Jackcina Stobel Christy E, Rajeswari A, Gopi S, Pius A (2020) Chitin and chitosan-based aerogels. Handbook of chitin and Chitosan. Elsevier, pp 285–334
Whitehead FA, Young SA, Kasapis S (2020) Swelling behaviour and glass transition in genipin-crosslinked chitosan systems. Int J Biol Macromol 164:3075–3083. https://doi.org/10.1016/j.ijbiomac.2020.08.178
Ray M, Pal K, Anis A, Banthia AK (2010) Development and characterization of chitosan-based polymeric hydrogel membranes. Des Monomers Polym 13:193–206. https://doi.org/10.1163/138577210X12634696333479
Bigi A, Cojazzi G, Panzavolta S et al (2002) Stabilization of gelatin films by crosslinking with genipin. Biomaterials 23:4827–4832. https://doi.org/10.1016/S0142-9612(02)00235-1
Perez V, Felix M, Romero A, Guerrero A (2016) Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod Process 97:100–108. https://doi.org/10.1016/j.fbp.2015.12.004
Gorczyca G, Tylingo R, Szweda P et al (2014) Preparation and characterization of genipin cross-linked porous chitosan-collagen-gelatin scaffolds using chitosan-CO2 solution. Carbohydr Polym 102:901–911. https://doi.org/10.1016/j.carbpol.2013.10.060
Felix M, Perez-Puyana V, Romero A, Guerrero A (2017) Development of protein-based bioplastics modified with different additives. J Appl Polym Sci 143:45430
Fernández-Espada L, Bengoechea C, Cordobés F, Guerrero A (2016) Protein/glycerol blends and injection-molded bioplastic matrices: soybean versus egg albumen. J Appl Polym Sci 133:42980. https://doi.org/10.1002/app.42980
Ma X, He Z, Han F et al (2016) Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids Surf B Biointerfaces 143:81–87. https://doi.org/10.1016/j.colsurfb.2016.03.025
Tang Y, Song W, Qiao J et al (2019) A study of corneal structure and biomechanical properties after collagen crosslinking with genipin in rabbit corneas. Mol Vis 25:574–582
Zhou X, Tao Y, Chen E et al (2018) Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J Biomed Mater Res Part A 106:1258–1268. https://doi.org/10.1002/jbm.a.36325
Yu Y, Xu S, Li S, Pan H (2021) Genipin-cross-linked hydrogels based on biomaterials for drug delivery: a review. Biomater Sci 9:1583–1597. https://doi.org/10.1039/D0BM01403F
Jiménez-Rosado M, Bouroudian E, Perez-Puyana V et al (2020) Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J Clean Prod 262:121517. https://doi.org/10.1016/j.jclepro.2020.121517
Acknowledgements
This work is a part of a I+D+i PID2021-124294OB-C21 project which is sponsored by MCIN/AEI/10.13039/501100011033/ and “ERDF A way of making Europe”. The authors thank their financial support. In addition, the authors would also acknowledge for the postdoctoral contract of Víctor M. Pérez Puyana from the “Contratación de Personal Investigador Doctor” supported by the European Social Fund and Junta de Andalucía (PAIDI DOCTOR – Convocatoria 2019-2020).
Funding
Funding for open access publishing: Universidad de Sevilla/CBUA.
Author information
Authors and Affiliations
Contributions
Conceptualization: VPP; methodology, ECT and MJR; investigation, VPP and ECT; resources, AR and IM; data curation, MJR.; writing—original draft preparation, VPP and ECT; visualization, IM; supervision, AR and IM; funding acquisition, AR. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perez-Puyana, V.M., Cortés-Triviño, E., Jiménez-Rosado, M. et al. Pea Protein-Based Bioplastics Crosslinked with Genipin: Analysis of the Crosslinking Evolution. J Polym Environ 32, 31–44 (2024). https://doi.org/10.1007/s10924-023-02973-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02973-0