Abstract
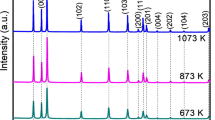
The use of environmentally benign alternatives to replace harmful petrochemical-based polymers has been largely explored. The work reports an eco-friendly polymeric system derived from vegetable oil (Corn oil) and evaluated the impact on the functional properties due to the variation in d-orbital electrons (Co/Cu). The dispersed transition metal (TM) acts as the connecting nuclei generating layered structures using the corn diol fatty amide (CDFA) ligands that facilitate the conversion of linear CDFA into crosslinked multifunctional metallopolymers (MP). The transmission electron microscopic images of CuXMPY show the presence of well-dispersed nanospheres (15 ± 8 nm), however, the images for CoXMPY reveal the formation of larger particles (ca. 100 nm). The incorporation of the respective TMs influences the physicomechanical, thermal, and electrochemical properties. The energy-dispersive X-ray and X-ray photoelectron spectroscopy studies confirm the presence of TMs in the metallopolymers (MPs). The XPS suggests a dominant interaction of polar “N” ends over “O” groups during the self-assembled coordination of polymers with the TM ions. The incorporation of TMs improves the crystalline nature, water contact angles (89° and 92°), and corrosion resistance abilities (electrochemical impedance performance) in the MPs. The work highlights thorough electrochemical studies (corrosion resistance, current, and potential values) and the importance of the TMs used during the development of functional and sustainable polymeric coating.
Graphical Abstract















Similar content being viewed by others
References
El-Sonbati AZ, El-Mogazy MA, Nozha SG, Diab MA, Abou-Dobara MI, Eldesoky AM, Morgan SM (2022) Mixed ligand transition metal(II) complexes: characterization, spectral, electrochemical studies, molecular docking and bacteriological application. J Mol Struct. https://doi.org/10.1016/j.molstruc.2021.131498
Lim KRG, Handoko AD, Nemani SK, Wyatt B, Jiang HY, Tang J, Anasori B, Seh ZW (2020) Rational design of two-dimensional transition metal carbide/nitride (MXene) hybrids and nanocomposites for catalytic energy storage and conversion. ACS Nano. https://doi.org/10.1021/acsnano.0c05482
Tamayo L, Azócar M, Kogan M, Riveros A, Páez M (2016) Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mate Sci Eng C. https://doi.org/10.1016/j.msec.2016.08.041
Honarvar Nazari M, Zhang Y, Mahmoodi A, Xu G, Yu J, Wu J, Shi X (2022) Nanocomposite organic coatings for corrosion protection of metals: a review of recent advances. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2021.106573
Ghosal A, Iqbal S, Ahmad S (2019) NiO nanofiller dispersed hybrid soy epoxy anticorrosive coatings. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2019.04.029
Fadl AM, Abdou MI, Laila D, Sadeek SA (2020) Fabrication and characterization of novel p-phenylamine-N(4-chloro salicylaldenemine) ligand and its metal complexes and evaluation their anti-corrosion and chemical resistance properties in epoxy/SiO2 nanocomposite for steel surface coating. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123390
Kitagawa S, Kitaura R, Noro SI (2004) Functional porous coordination polymers. Angew Chem Int Ed. https://doi.org/10.1002/anie.200300610
Moroi GN (2009) Preparation and characterization of polyesterurethane metallopolymers containing transition metal ions. Macromol Symp. https://doi.org/10.1002/masy.200950505
Ahmadi M, Zabihi O, Jeon S, Yoonessi M, Dasari A, Ramakrishna S, Naebe M (2020) 2D transition metal dichalcogenide nanomaterials: advances, opportunities, and challenges in multi-functional polymer nanocomposites. J Mater Chem A Mater. https://doi.org/10.1039/c9ta10130f
Pourhashem S, Saba F, Duan J, Rashidi A, Guan F, Nezhad EG, Hou B (2020) Polymer/inorganic nanocomposite coatings with superior corrosion protection performance: a review. J Ind Eng Chem 88:29–57. https://doi.org/10.1016/j.jiec.2020.04.029
Teijido R, Ruiz-Rubio L, Echaide AG, Vilas-Vilela JL, Lanceros-Mendez S, Zhang Q (2022) State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2021.106684
Mukherjee S, Sharma S, Ghosh SK (2019) Hydrophobic metal-organic frameworks: potential toward emerging applications. APL Mater 10(1063/1):5091783
Pourhashem S, Seif A, Saba F, Nezhad EG, Ji X, Zhou Z, Zhai X, Mirzaee M, Duan J, Rashidi A, Hou B (2022) Antifouling nanocomposite polymer coatings for marine applications: a review on experiments, mechanisms, and theoretical studies. J Mater Sci Technol. https://doi.org/10.1016/j.jmst.2021.11.061
Suraj Belgaonkar M, Kandasubramanian B (2021) Hyperbranched polymer-based nanocomposites: synthesis, progress, and applications. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2021.110301
Masood S, Ghosal A, Gupta A, Zafar F, Kumari R, Alam M, Nishat N (2022) Comparative studies on coating materials of urotropine modified furfurylolated-tCNSL and methylolated-tCNSL thermoset for anticorrosive application: switching towards a cleaner approach. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.130933
Zafar F, Ghosal A, Sharmin E, Chaturvedi R, Nishat N (2019) A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog Org Coat 131:259–275. https://doi.org/10.1016/j.porgcoat.2019.02.014
Jaya Prakash N, Kandasubramanian B (2021) Nanocomposites of MXene for industrial applications. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2020.158547
Yashas SR, Shahmoradi B, Wantala K, Shivaraju HP (2021) Potentiality of polymer nanocomposites for sustainable environmental applications: a review of recent advances. Polymer. https://doi.org/10.1016/j.polymer.2021.124184
Idumah CI (2021) Novel advancements in green and sustainable polymeric nanocomposites coatings. Curr Res Green Sustain Chem. https://doi.org/10.1016/j.crgsc.2021.100173
Khan K, Tareen AK, Aslam M, Zhang Y, Wang R, Ouyang Z, Gou Z, Zhang H (2019) Recent advances in two-dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale. https://doi.org/10.1039/c9nr05919a
Aghamohammadi H, Amousa N, Eslami-Farsani R (2021) Recent advances in developing the MXene/polymer nanocomposites with multiple properties: a review study. Synth Met. https://doi.org/10.1016/j.synthmet.2020.116695
Dayan S, Ozpozan NK, Özdemir N, Dayan O (2014) Synthesis of some ruthenium(II)-Schiff base complexes bearing sulfonamide fragment: new catalysts for transfer hydrogenation of ketones. J Organomet Chem. https://doi.org/10.1016/j.jorganchem.2014.08.002
Laxmi S, Khan A, Kareem F, Zafar N, Nishat (2018) Synthesis, vibrational spectrometry and thermal characterizations of coordination polymers derived from divalent metal ions and hydroxyl terminated polyurethane as ligand. Spectrochim Acta A Mol Biomol Spectrosc 188:400–410. https://doi.org/10.1016/j.saa.2017.07.032
Ghiyasiyan-Arani M, Masjedi-Arani M, Ghanbari D, Bagheri S, Salavati-Niasari M (2016) Novel chemical synthesis and characterization of copper pyrovanadate nanoparticles and its influence on the flame retardancy of polymeric nanocomposites. Sci Rep. https://doi.org/10.1038/srep25231
Xue B, Li F, Xing Y, Sun M, Liu D, Jiang Y (2011) Preparation of Cu/OMMT/LLDPE nanocomposites and synergistic effect study of two different nano materials in polymer matrix. Polym Bull. https://doi.org/10.1007/s00289-011-0466-3
El-Lateef HMA, Gouda M (2021) Novel nanocomposites of nickel and copper oxide nanoparticles embedded in a melamine framework containing cellulose nanocrystals: material features and corrosion protection applications. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116960
Alam M, Alandis NM, Ahmad N, Husain FM (2020) Anticorrosive and antibacterial nanocomposite coating material from sustainable resource. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2020.112955
Alam M, Alandis NM, Ahmad N (2017) Development of poly(urethane-ester)amide from corn oil and their anticorrosive studies. Inter J Polym Anal Charact. https://doi.org/10.1080/1023666X.2017.1287847
Zafar F, Zafar H, Sharmin E, Ahmad S (2010) Studies on self cured zinc-containing Pongamia glabra oil based polyesteramide. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2010.09.008
Zafar F, Mir MH, Kashif M, Sharmin E, Ahmad S (2011) Microwave assisted synthesis of Bio based metallopolyurethaneamide. J Inorg Organomet Polym Mater 21:61–68
Ahmad S, Ashraf SM, Naqvi F, Yadav S, Hasnat A (2003) A polyesteramide from Pongamia glabra oil for biologically safe anticorrosive coating. Prog Org Coat 47:95–102. https://doi.org/10.1016/S0300-9440(03)00015-8
Paraskar PM, Prabhudesai MS, Hatkar VM, Kulkarni RD (2021) Vegetable oil based polyurethane coatings–a sustainable approach: a review. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2021.106267
Pouladi J, Mirabedini SM, Eivaz Mohammadloo H, Rad NG (2021) Synthesis of novel plant oil-based isocyanate-free urethane coatings and study of their anti-corrosion properties. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2021.110502
Sharmin E, Zafar F, Akram D, Alam M, Ahmad S (2015) Recent advances in vegetable oils based environment friendly coatings: a review. Ind Crops Prod 76:215–229. https://doi.org/10.1016/j.indcrop.2015.06.022
Zafar F, Zafar H, Sharmin E, Ahmad S (2010) Studies on self cured zinc-containing Pongamia glabra oil based polyesteramide. Prog Org Coat 69:517–521
Hu P, Xie Q, Ma C, Zhang G (2021) Fouling resistant silicone coating with self-healing induced by metal coordination. Chem Eng J. https://doi.org/10.1016/j.cej.2020.126870
Alam M, Alandis NM, Zafar F, Sharmin E, Al-Mohammadi YM (2018) Polyurethane-TiO2 nanocomposite coatings from sunflower- oil-based amide diol as soft segment. J Macromol Sci Part A Pure Appl Chem. https://doi.org/10.1080/10601325.2018.1526638
Umoren SA, Solomon MM (2017) Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2016.12.001
Ghosal A, Ahmad S (2017) High performance anti-corrosive epoxy-titania hybrid nanocomposite coatings. New J Chem 41:4599–4610. https://doi.org/10.1039/c6nj03906e
Alam M, Alandis NM, Ahmad N, Husain FM (2020) Anticorrosive and antibacterial nanocomposite coating material from sustainable resource. Ind Crops Prod 158:112955. https://doi.org/10.1016/j.indcrop.2020.112955
Kashif M, Sharmin E, Zafar F, Ahmad S (2011) Synthesis and characterization of ricinoleamide-based polyurethane. JAOCS 88:1989–1996
Ahmad S, Zafar F, Sharmin E, Garg N, Kashif M (2012) Synthesis and characterization of corrosion protective polyurethanefattyamide/silica hybrid coating material. Prog Org Coat 73:112–117. https://doi.org/10.1016/j.porgcoat.2011.09.007
Chen WC, Chen HH, Wen TC, Digar M, Gopalan A (2004) Morphology and ionic conductivity of thermoplastic polyurethane electrolytes. J Appl Polym Sci. https://doi.org/10.1002/app.13208
Alam M, Zafar F, Ghosal A, Ahmed M (2022) Formulation of silica-based corn oil transformed polyester acryl amide-phenol formaldehyde corrosion resistant coating material. J Appl Polym Sci. https://doi.org/10.1002/app.51651
Ramezanzadeh B, Haeri Z, Ramezanzadeh M (2016) A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem Eng J. https://doi.org/10.1016/j.cej.2016.06.028
Ghosal A, Shah J, Kotnala RK, Ahmad S (2013) Facile green synthesis of nickel nanostructures using natural polyol and morphology dependent dye adsorption properties. J Mater Chem A Mater. https://doi.org/10.1039/c3ta12716h
Pourhashem S, Saba F, Duan J, Rashidi A, Guan F, Nezhad EG, Hou B (2020) Polymer/inorganic nanocomposite coatings with superior corrosion protection performance: a review. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2020.04.029
Zafar F, Ashraf SM, Ahmad S (2008) In situ development of Zn/Cd-incorporated poly(esteramide-urethane) from sustainable resource. J Appl Polym Sci 110:584–593
Lee KJ, Lee DK, Kim YW, Choe WS, Kim JH (2007) Theoretical consideration on the glass transition behavior of polymer nanocomposites. J Polym Sci B Polym Phys. https://doi.org/10.1002/polb.21178
Prajitno DH, Maulana A, Syarif DG (2016) Effect of surface roughness on contact angle measurement of nanofluid on surface of stainless steel 304 by sessile drop method. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/739/1/012029
Cieśliński JT, Krygier KA (2014) Sessile droplet contact angle of water-Al2O3, water-TiO2 and water-Cu nanofluids. Exp Therm Fluid Sci. https://doi.org/10.1016/j.expthermflusci.2014.06.004
Li G, Liu B, Bai L, Shi Z, Tang X, Wang J, Liang H, Zhang Y, Van der Bruggen B (2020) Improving the performance of loose nanofiltration membranes by poly-dopamine/zwitterionic polymer coating with hydroxyl radical activation. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2019.116412
Mahdavian M, Attar MM (2009) Electrochemical behaviour of some transition metal acetylacetonate complexes as corrosion inhibitors for mild steel. Corros Sci. https://doi.org/10.1016/j.corsci.2008.11.010
Cheng L, Wu H, Li J, Zhao H, Wang L (2021) Polydopamine modified ultrathin hydroxyapatite nanosheets for anti-corrosion reinforcement in polymeric coatings. Corros Sci. https://doi.org/10.1016/j.corsci.2020.109064
Acknowledgements
The authors are grateful to the Researchers Supporting Project (RSP2023R113), King Saud University, Riyadh, Saudi Arabia for the support.
Funding
Researchers Supporting Project (RSP2023R113).
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to this research work.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alam, M., Ghosal, A., Zafar, F. et al. Preparation of Co(II)/Cu(II) Metal-Based Metallopolymer Nanocomposites: A Protective Coating for Carbon Steel. J Polym Environ 32, 588–606 (2024). https://doi.org/10.1007/s10924-023-02968-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02968-x