Abstract
In this work, two catalysts based on polyethylene glycol (PEG) containing sulfonic acid group and the −COOH group of citric acid (CA) were synthesized. Characterization of the −SO3H functionalized PEG and citric acid functionalized PEG has been carried out using FT–IR. The acidity of PEG–SO3H and PEG–CA has been explored to investigate their catalytic efficiency towards eco-friendly production of methyl salicylate via esterification of salicylic acid using methanol as a reactant as well as solvent. Methanol to acid molar ratios of 4:1, 6:1, 8:1, and 10:1 was applied. The sulfonated PEG is found to be a very active solid acid catalyst giving high yields (82%) under the optimized reaction conditions (10:1 M ratio of methanol to acid; reaction temperature, 65 °C; reaction time, 150 min with catalyst loading of 1.5%. In comparison with the catalytic activity of H2SO4, PEG–SO3H, and PEG-CA; the PEG–SO3H surpassed the catalytic activity of both H2SO4 and PEG–CA. The IEC of PEG–SO3H was 4 meq/g. And the maximum water uptake of PEG-SO3H was 8.25%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esterification is an important reaction for the production of several chemicals which have significant value in different industries such as pharmaceuticals, solvents plasticizers, etc. [1]. Methyl salicylate is an ingredient of wintergreen and other plants like Amblyomma veriegatum, and it’s utilized as a local anesthetic and disinfectant in toothpaste, mouthwash, fragrances, and flavoring agents. Methyl salicylate is utilized for a variety of medical purposes, including the treatment of muscle discomfort [2]. Former researchers have demonstrated the use of homogeneous and heterogeneous catalysts. An important issue about the esterification reaction is that an acid-based catalyst should be used to shift the equilibrium in the forward direction. However homogeneous catalysts were found to cause corrosion problems in addition to an enormous amounts of toxic effluents [3,4,5]; and because the recovery of homogeneous catalysts was found to be expensive, heterogeneous catalysts were found to be a suitable alternative [6].
Many materials such as Zeolite, heteropolyacids, carbon-based solid acids, and zinc lanthanum mixed oxides have been documented to catalyze the esterification process [7]. Many researchers have focused on the use of ion-exchange resins as catalysts in the past few years. Polymer-supported catalysts have the benefit of being able to manipulate their textural characteristics by changing the synthesis conditions. Furthermore, this type of material is less toxic in comparison with metal catalysts, as it is not susceptible to leaching [8,9,10]. Larger surface areas in porous polymer particles result in a greater number of catalytic sites, therefore controlling this characteristic as a function of polymerization factors is of significant importance [11]. Organic polymers with appropriate functional groups at the pore surface are extremely required and have a wide range of applications, including drug delivery, adsorption, and catalysis [12]. Ethylene glycol (EG) is a common chemical resource that may be made from sustainable biomass. Furthermore, EG is an odorless, non-volatile, and low-toxic solvent with a wide range of industrial uses [13]. In the production of cyclic carbonates, PEGs have been considered simple phase-transfer catalysts [14, 15]. PEGs combine the benefits of homogeneous catalysis, such as high reactivity, lack of diffusion phenomena, and ease of analysis [16]. Green chemistry principles now motivate researchers to use solid acids with sulfonate groups functionalized solid acids as high-performance, reusable, and environmentally friendly catalysts for chemical syntheses, as green alternatives to toxic and unrecyclable homogeneous acid catalysts [17, 18]. catalysts containing –SO3H have received considerable attention as environmentally benign and can be used in organic synthesis particularly in the esterification and transesterification of various alcohols due to their higher acidic strength, increased thermal stability, and reduced toxicity [19,20,21,22]. The citric acid (CA) is an organic acid with a carboxylic group-rich structure that is inexpensive and ecologically benign. CA has recently been shown to serve a role comparable to that of typical catalysts employed in diverse organic transformations in many publications [23, 24]. There have been several studies on commercial sulfonated resin-catalyzed esterification methods [25, 26]. The purpose of the present study is to synthesize non-toxic, environmentally-friendly PEG–SO3H and PEG-CA catalysts and study the role of sulphonic and carboxylic groups in enhancing the surface properties and catalytic activity of PEG. Because of the use and relevance of PEG, the current study focused on its functionalization and usage in the production of methyl salicylate ester.
Materials and Methods
Materials
Ether, Salicylic Acid (C7H6O3), sodium chloride (purity 99.5%), sodium hydroxide (purity 96%), Polyethylene Glycol 6000, Methylene Chloride (CH2Cl2), Citric Acid anhydrous (C6H8O7), and methanol (purity 99.8%) were purchased from Sigma Aldrich, Egypt. Oleum (30%, Sigma Aldrich) was used as a sulfonating agent.
Synthesis of CA/PEG Catalyst
Citric acid was mixed with 1 g of PEG and the percentage of the citric acid was modified to produce catalysts containing 15% citric acid by weight. The mixture was stirred for 1.5 h to make a slurry. It was kept in a hot plate at 150 °C for 2 h to aid the thermochemical reaction. During this samples were withdrawn and Sodium Carbonate was added to the product to determine if there are any carboxylic acid groups left until no effervescence exists which signified the complete discharge of excess citric acid. Finally, the catalysts were dried for 2 h in a rotating oven at 100 °C.
Preparation of Sulfonated PEG Catalyst
A 60-g of PEG was dissolved in 100 ml of dichloroethane at room temperature. The sample has been placed in an ice bath. With stirring, oleum (H2SO4.SO3) was slowly added. At room temperature, the resultant solution was agitated for 16–20 h. The solution was then concentrated under a vacuum before being mixed with ether. PEG–OSO3H was obtained as a gummy solid after filtering the precipitate and washing it three times with ether.
General Procedure for Esterification of Salicylic Acid with Methanol Methyl Salicylate (Wintergreen Oil) in the Presence of a Catalyst
In a typical procedure, the prepared catalyst was tested by investigation of the salicylic acid and methanol in the molar ratio of 1:10 in a 350 ml round bottom flask, equipped with a magnetic stirrer and condenser, placed on a hot plate with magnetic stirring; the used setup is shown in Fig. 1. The activated catalysts are H2SO4 (as a Standard Sample), Sulfonated PEG, and PEG-Citrate (salicylic acid to catalyst molar weight ratio = 1000:1) were added to the reaction mixture each in a separate experiment and the reaction mixture was continuously heated on a hot plate with a magnetic stirrer at a temperature ranging from 60 to 65 °C and with a condenser. Samples were taken every 30 min to analyze the effect of each catalyst on the concentration of methyl salicylate in each period of time. Yields were determined by GC. In the end, as a final step, the reaction mixture was cooled and filtered to separate the catalyst. IR spectra of the reaction products were recorded on a Hitachai U2900 spectrophotometer in the region of 190–1100 nm with a resolution of 1.5 nm.
Characterization of Prepared Catalysts
Degree of Sulfonation
Using the acid–base titration technique, the sulfonation degree was estimated. This was carried out by immersing sulfonated PEG (0.1 g) into 10 mL of NaCl 0.1 M solution for 48 h. The filtrate was collected after separation and titrated with NaOH (0.02 M). The sulfonation degree is obtained from Eq. 1 [27]
Ion Exchange Capacity
Titration was used to estimate the ion exchange capacity (IEC), which was calculated in meq g−1. The active sites of a dry polymer (0.1 g) were protonated in a 1 M HCl solution for 24 h before being rinsed with distilled water to eliminate excess acid. The sample was then soaked for 24 h in a 1 M NaCl solution before being titrated with 0.01 M NaOH. The following equation was used to determine the ion exchange capacity:
where VNaOH is the volume (L) of NaOH, CNaOH is the Na+ ion concentration and m is the mass (g) of dry polymer. The ion exchange capacity estimates the density of the ionizable functional group in the polymer, responsible for the charge [27].
Water Uptake
Water uptake was determined by weighing the material before and after immersion in deionized water at room temperature for 24 h. The water uptake of the polymer is reported in wt% according to Eq. 3:
where w = weight of the polymer [27].
Fourier Transform Infrared Spectroscopy
IR spectra of the catalyst samples were recorded using a Fourier transform infrared spectrometer (FT–IR) Agilent Cary 630 FTIR.
Product Analysis
The samples were analyzed in the UV spectrophotometer by taking a 0.1 ml sample and diluting it with 9 ml methanol. The conversion was calculated as follows.
Results and Discussion
Characterization of Catalysts
Fourier Transform Infrared Spectroscopy of Sulfonated PEG Catalyst
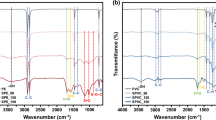
In this study, FTIR spectroscopy was used to confirm the chemical structure of the sulfonated PEG using convenient calculation of the functional groups. Characteristic absorptions observed are shown in Figs. 2 and 3. The successful inclusion of the sulfonic acid group was confirmed by the FTIR spectra of sulfonated PEG as illustrated in Fig. 1, where the strong characteristic peak at 1073.3 cm−1 is assignable to the sulfonic acid group. The peaks around 2873.3cm−1 are attributed to the alkyl chain of polymer; the band at 1348.2 is due to C–H bending and C–O stretching vibration, respectively; and the band at1246.3 cm−1 corresponds to C–H twisting vibrations [28].
Fourier Transform Infrared Spectroscopy of PEG citrate Catalyst
The successful introduction of the carboxyl functional group was confirmed by the FTIR spectra of PEG- citrate as illustrated in Fig. 4. Vibration frequencies at 1730 cm–1 (ν(C=O) s) were noticed, characteristics of the carboxyl functional groups on the COOH-PEG [23].
Water Uptake, Exchange Capacity, and Degree of Sulphonation (DS)
The SPEG had an 8.25% water uptake capacity, which is attributed to the introduction of sulfonic groups in the polyethylene glycol. Such –SO3H groups have a polar nature which stimulates water uptake. This impact might be due to a greater ionic interaction, which increased the sulfonated polymer's cross-linking density, allowing only limited water uptake. The catalytic activity rises with the increase of acid site number and water absorption capacity. Calculated IEC values show that –SO3H is the most reactive sulfonic group [27]. The ion exchange capacity value for SPEG was 4 meq g−1, which is in the same range for previously studied catalysts, namely 3–5 meq g−1 [29].
The degree of sulfonation (DS) indicates the amount of sulfonated groups per repeating unit is regulated by changing the monomer-to-monomer ratio. This is also observed by FT-IR analysis. It has been observed that by extending the time of acid treatment the sulfonation degree increases. The calculated degree of sulfonation was 26% [30].
Comparison of the Performance of Different Catalysts
The progress of the esterification of salicylic acid was followed by UV–Vis spectroscopy. The main absorption bands are located at 292.5 nm as shown in Fig. 5.
On the esterification of salicylic acid, Fig. 6 examines the effects of three different catalysts. The salicylic acid conversion was found to be between 32 and 82 percent for all catalysts.
The inclusion of a sulfonic acid group in the SPEG catalyst results in greater catalytic activity, indicating that the acidity of the catalyst is a key role in conversion [31]. The salicylic acid concentration increase after 1.5 hour when using citrate as a catalyst which may be due to hydrolysis of the formed methyl salicylate. Because the esterification reaction is reversible, increasing the duration beyond the optimum allows the reaction to proceed in the opposite direction, resulting in a rise in acid value which agrees with the work of [32]. The results of the two catalysts were compared with sulfuric acid and revealed that SPEG catalysts give promising results.
Effect of the Stirring Speed
From 100 to 400 rpm at 64 °C, the influence of external mass transfer resistance on the conversion of salicylic acid was investigated for a PEG-SO3H catalyst loading of 1%. (Fig. 7). At 400 rpm, the greatest conversion of salicylic acid was recorded, indicating that there was no external mass transfer resistance in the process. Stirring keeps the reactant particles moving, increasing the odds of collision and the reaction rate. As a result, all subsequent trials were run at 400 rpm.
Effect of Catalyst Loading
The catalyst loading effect was analyzed from 0.5 to 2% (Fig. 8). Due to the linear rise in the number of active sites, conversion improves with an increase in catalyst ratio up to 1%. The minimal catalyst loading resulted in an inadequate number of active sites to catalyze the reaction. As it was expected, the conversion was increased from 50 to 82% by increasing the catalyst concentration from 0.5% to 2 wt%.
Furthermore, it seems that higher concentrations beyond 1.5% caused limitations on mass transfer. The results agree with similar work by Bhusari et al. [19].
Effect of Changing the Salicylic Acid to Methanol Mole Ratio
Salicylic acid to methanol mole ratio was varied by changing of solvent: acid ratio, using ratios (1:4–1:6–1:8–1:10); under similar conditions by keeping the total volume constant (Fig. 9). The increase in salicylic acid conversion was attributed to an increase in the number of moles of methanol concerning salicylic acid. However, a 1:10 mol ratio was taken for further experiments. A higher alcohol ratio was observed to be capable of driving the reaction forward more efficiently. We take alcohol in more amounts because it is cheap compared to acid. According to Le chatelier principle taking one reactant in excess amount to precede reaction forward. In most instances, when the actual esterification is concerned, the surplus occurrence of one component can make the reaction proceed well. Therefore, when the methyl alcohol ratio occurs in excess, more ester is created by a shifted pattern of esterification [33]. These results obtained were united in opinion with a previous studies [34].
Effect of Reaction Time
Another important factor that influences the ultimate product yield is reaction time. Each experiment was run at MeOH: SA molar ratio of 10:1, with 1.5 wt% loading of the catalyst at a temperature of 65 °C. The effect of reaction time (30 to 150 min) was depicted in Fig. 10. At the first 30 min of the reaction, the yield of methyl ester was 70% which was enhanced from 72% while the reaction time was prolonged from 30 to 60 min. As the esterification was carried out for 150 min, the yield reached its highest value of 81.7%. The yield remained nearly constant after extending the reaction beyond 90 min. From the results, the highest yield was achieved at 150 min of the esterification over the SPEG catalyst.
Mechanism of the Esterification System
The reaction of salicylic acid with methanol is a nucleophilic substitution that is so slow that it needed to be accelerated by high temperature or a catalyst to enhance the overall reaction. Usage of higher temperature would leads to evaporation of the liquid and hence loss of reactants and also increase in the reactor pressure which means higher cost of methyl salicylate production. A possible mechanism of methyl salicylate production was illustrated in Fig. 11, which illustrates the nucleophilic attack by methanol on carboxy. The observed action could be illustrated as follows: On the surface of the functionalized catalyst, SA was absorbed on the acid sites by its carbonyl oxygen, and then the methanol attacked the unstabilizing cationic intermediate. After the electron transfer reaction took place, the methyl salicylate is formed [35].
Conclusion
Polyethylene glycol was successfully sulfonated using oleum. Evidence of sulfonation both qualitative and quantitative was provided by the FTIR and degree of sulfonation DS, also cation exchange capacity (CEC) and water uptake.
Esterification of salicylic acid with methyl alcohol was carried out using polyethylene glycol (PEG) functionalized by sulfonic acid group, and the −COOH group of citric acid (CA), and they were compared with H2SO4. The results proved that SPEG was the most active catalyst in the esterification of salicylic acid, due to increase in acidic sites. The highest salicylic acid conversion of 82% was obtained with a mole ratio of 1:10 of salicylic acid to methanol at 65 °C for 150 min at a catalyst loading of 1.5%. The optimal conditions were determined by examining several factors such as catalyst loading, stirring speed, and the mole ratio of salicylic acid to methanol.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bhusari AA, Mazumdar B, Rathod AP, Khonde RD (2020) Kinetics of catalyzed esterification of acetic acid with n-butanol using carbonized agro-waste. Int J Chem Kinet 52(7):450–462
Mendes S, Catarino A, Zille A, Fernandes N, Bezerra FM (2021) Vehiculation of methyl salicylate from microcapsules supported on textile matrix. Materials 14(5):1087
Chandane VS, Rathod AP, Wasewar KL, Sonawane SS (2018) Synthesis of cenosphere supported heterogeneous catalyst and its performance in esterification reaction. Chem Eng Commun. https://doi.org/10.1080/00986445.2017.1384922
Chandane VS, Rathod AP, Wasewar KL (2019) Pervaporation-assisted esterification of caproic acid with isobutanol in conventional, in situ, and ex situ reactors. Chem Eng Technol. https://doi.org/10.1002/ceat.201800506
Chandane VS, Rathod AP, Wasewar KL, Jadhav PG (2020) Response surface methodology and artificial neural networks for optimization of catalytic esterification of lactic acid. Chem Eng Technol 43(11):2315–2324
Zhang J, Liu J, Huang X, Choi S, Zhu R, Tang S et al (2020) Heterogeneous catalytic esterification of oleic acid under sub/supercritical methanol over γ-Al2O3. Fuel 268:117359
Zhou Y, Noshadi I, Ding H, Liu J, Parnas RS, Clearfield A et al (2018) Solid acid catalyst based on single-layer α-zirconium phosphate nanosheets for biodiesel production via esterification. Catalysts 8(1):17
Penariol JL, Theodoro TR, Dias JR, Carpegiani JA, Aguiar LG (2019) Application of a sulfonated styrene–(ethylene glycol dimethacrylate) resin as catalyst. Kinet Catal 60(5):650–653
Sand H, Weberskirch R (2017) Bipyridine copper functionalized polymer resins as support materials for the aerobic oxidation of alcohols. Polym Int 66(3):428–435
Foss LE, Shabalin KV, Musin LI, Nagornova OA, Salikhov RZ, Borisov DN, Musin RZ, Yakubov MR (2020) Synthesis of asphaltene-based strongly acidic sulfonated cation exchangers and determination of their catalytic properties in the 2, 2-dimethyl-1, 3-dioxolane synthesis reaction. Pet Chem 60(6):709–715
Aguiar LG, Moura JO, Theodoro TR, Neto TG, Lopes VM, Dias JR (2017) Prediction of resin textural properties by vinyl/divinyl copolymerization modeling. Polymer 129:21–31
Gomes R, Bhanja P, Bhaumik A (2016) Sulfonated porous organic polymer as a highly efficient catalyst for the synthesis of biodiesel at room temperature. J Mol Catal A 411:110–116
Jiang YQ, Li J, Feng ZW, Xu GQ, Shi X, Ding QJ et al (2020) Ethylene glycol: a green solvent for visible light-promoted aerobic transition metal-free cascade sulfonation/cyclization reaction. Adv Synth Catal 362(13):2609–2614
Steinbauer J, Werner T (2017) Poly (ethylene glycol) s as ligands in calcium-catalyzed cyclic carbonate synthesis. Chemsuschem 10(15):3025–3029
Soni J, Sahiba N, Sethiya A, Agarwal S (2020) Polyethylene glycol: a promising approach for sustainable organic synthesis. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.113766
Yang LJ, Luo JG, Wang XC (2017) PEG-OSO3H as an efficient catalyst in the synthesis of n3-functionalized 3.4-dihydropyrimidinone and quinazolinone derivatives. Heterocycles 94(6):1027–1039
Rather RA, Khan MU, Siddiqui ZN (2020) Sulphated alumina tungstic acid (SATA): a highly efficient and novel heterogeneous mesostructured catalyst for the synthesis of pyrazole carbonitrile derivatives and evaluation of green metrics. RSC Adv 10(2):818–827
Yue X, Wu Z, Wang G, Liang Y, Sun Y, Song M et al (2019) High acidity cellulose sulfuric acid from sulfur trioxide: a highly efficient catalyst for the one step synthesis of xanthene and dihydroquinazolinone derivatives. RSC Adv 9(49):28718–28723
Bhusari AA, Mazumdar B, Rathod AP (2021) Sulphonated tea waste carbon catalyzed esterification of propionic acid with ethyl alcohol: modulus and rate constant estimation. Waste Biomass Valoriz 12(3):1303–1312
Bhusari AA, Mazumdar B, Rathod AP, Marghade D (2020) Catalytic aspect of biomass in microcontroller-assisted esterification. Energy Sources Part A 24:1–16
Heirati SZD, Shirini F, Shojaei AF (2017) Sulfonated PEG-intercalated montmorillonite [(Mt/PEG)-SO 3 H] as efficient and ecofriendly nanocatalyst for synthesis of α, α′-bis (substituted benzylidene) cycloalkanones. Res Chem Intermed 43(11):6167–6186
Jiao L, Sun S, Meng X, Ji P (2019) Sn-based porous coordination polymer synthesized with two ligands for tandem catalysis producing 5-hydroxymethylfurfural. Catalysts 9(9):739
Maleki A, Hajizadeh Z, Abbasi H (2018) Surface modification of graphene oxide by citric acid and its application as a heterogeneous nanocatalyst in organic condensation reaction. Carbon letters 27:42–49
Safaei-Ghomi J, Tavazo M, Vakili MR, Shahbazi-Alavi H (2017) Chitosan functionalized by citric acid: an efficient catalyst for one-pot synthesis of 2, 4-diamino-5 H-[1] benzopyrano [2, 3-b] pyridine-3-carbonitriles 5-(arylthio) or 5-[(arylmethyl) thio] substituted. J Sulfur Chem 38(3):236–248
Silva VFL, Penariol JL, Dias JR, Theodoro TR, Carpegiani JA, Aguiar LG (2019) Sulfonated styrene-dimethacrylate resins with improved catalytic activity. Kinet Catal 60(5):654–660
Samorì C, Parodi A, Tagliavini E, Galletti P (2021) Recycling of post-use starch-based plastic bags through pyrolysis to produce sulfonated catalysts and chemicals. J Anal Appl Pyrol 155:105030
Ngadiwiyana N, Prasetya NBA, Gunawan G, Kusworo TD, Susanto H (2021) Synthesis, characterization, and study of proton exchange polymer membrane properties of sulfonated copolymer eugenol-diallyl Phthalate. Indonesian J Chem 21(1):168–178
Sundararajan S, Kumar A, Chakraborty BC, Samui AB, Kulkarni PS (2018) Poly (ethylene glycol)(PEG)-modified epoxy phase-change polymer with dual properties of thermal storage and vibration damping. Sustain Energy Fuels 2(3):688–697
De Leon-Condes CA, Roa-Morales G, Martinez-Barrera G, Balderas-Hernandez P, Menchaca-Campos C, Urena-Nunez F (2019) A novel sulfonated waste polystyrene/iron oxide nanoparticles composite: green synthesis, characterization and applications. J Environ Chem Eng 7(1):102841
Burak Y, Ali MK, Karataş B, Gürsel SA, Karataş Y (2022) Synthesis and characterization of poly (m-tolyloxy-co-4-pyridinoxy phosphazene) s and their application as proton exchange membranes. ChemistrySelect 7(3):e202103650
Shao Y, Li Q, Dong X, Wang J, Sun K, Zhang L et al (2021) Cooperation between hydrogenation and acidic sites in Cu-based catalyst for selective conversion of furfural to γ-valerolactone. Fuel 293:120457
Saha R, Goud VV (2015) Ultrasound assisted transesterification of high free fatty acids karanja oil using heterogeneous base catalysts. Biomass Conversion and Biorefinery 5(2):195–207
Sundar AM, Dharmendirakumar M (2022) Biodiesel production and comparison using commercial lipase and chemical catalyst from Cassia auriculata oil. J Indian Chem Soc 99(1):100265
Ibrahim SM (2021) Preparation, characterization and application of novel surface-modified ZrSnO4 as Sn-based TMOs catalysts for the stearic acid esterification with methanol to biodiesel. Renewable Energy 173:151–163
Bhaskaran RP, Nayak KH, Babu BP (2021) Synthesis of functionalized benzo [1, 3] dioxin-4-ones from salicylic acid and acetylenic esters and their direct amidation. RSC Adv 11(40):24570–24574
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). All authors declare that no funding was received.
Author information
Authors and Affiliations
Contributions
RF Conceived and designed the analysis; Collected the data; Performed the analysis; Wrote the paper; YS contributed data and analysis tools.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare there is no Conflicts of interest/Competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farouq, R., Selim, Y. Functionalized Polyethylene Glycol as a Catalyst for Esterification of Salicylic Acid. J Polym Environ 31, 2285–2293 (2023). https://doi.org/10.1007/s10924-022-02754-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02754-1