Abstract
Designing adsorbent materials that can effectively remove many types of organic dyes is crucial because of the wide diversity of synthetic dyes found in wastewater. Thus, this study presents the synthesis of economic and eco-friendly adsorbent composites composed of chitosan (Cs), talc (T), and Cloisite 30B clay (C) to remove both cationic crystal violet (CV) and anionic reactive yellow 145 (RY) dyes for the first time. Cs was functionalized with T and subsequently the CsTC1 and CsTC2 composites were prepared by sensitizing the obtained CsT with different weight ratios of Cloisite 30B (C). X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), thermogravimetric analysis (TGA), N2 adsorption–desorption isotherm, and zeta potential analysis were employed to characterize the materials. The addition of T to Cs matrix improves the thermal stability, pore size, and pore volume with respect to pure Cs. The effect of C inclusion was examined by measuring the affinity of the prepared composites towards adsorption of cationic CV and anionic RY dyes in comparison to pure Cs and CsT composite. The adsorption results in a single dye solution revealed that the CsTC1 composite is the most effective adsorbent for removal of RY dye with 76.9 mg/g adsorption capacity, whereas the CsTC2 composite exhibited the highest adsorption activity for CV dye (37.03 mg/g). Furthermore, RY and CV co-adsorption on CsTC composites was tested from a mixed dye solution. The adsorption kinetics of RY and CV adsorption followed the pseudo second order model. Langmuir isotherm model described the experimental adsorption data better than the Freundlich, Dubinin–Radushkevich, and Temkin isotherm models, indicating a monolayer sorption process for both dyes. The proposed mechanism for RY and CV adsorption using CsTC composites was investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, remediation of contaminated water from synthetic dyes is considered one of the most important challenges facing all countries due to the harmful effects of these dyes on the environment [1, 2]. Synthetic dyes are found in wastewater as a result of improper discharge of dyes from the textile, leather, paper, rubber, plastics, and pharmaceuticals industries [3, 4]. The presence of these dyes in the wastewater stream can cause a variety of environmental issues because they are carcinogenic and mutagenic to living organisms even at low concentrations [5, 6]. For example crystal violet (CV) dye, belongs to cationic dyes, which is frequently employed in textile factories to color cotton and silk, as well as in the manufacture of paints and inks. CV dye is employed to recognize bloody fingerprints [7]. CV may cause mild irritation of the eyes and serious damage to the conjunctiva and cornea. Exposure to CV in excess may cause harm to the digestive tract and skin, as well as kidney and respiratory problems. In addition, CV is non-biodegradable and is hardly ever digested by microorganisms. As a result, it can persist in a variety of conditions [8]. Consequently, the elimination of CV from solution is crucial and needed as will be discussed in this study in addition to reactive yellow 145 dye. Since it was frequently used in textile industries’ dying procedures and can be detected in industrial effluent. Therefore, the removal of these dyes before they are discharged into various water bodies has become an essential step [9]. Several methods have been tested for removing toxic dyes from wastewater, such as biological, ion exchange, advanced oxidation, coagulation, flocculation, ultra-filtration through membranes, and adsorption methods [3, 6, 10]. Adsorption method has received great attention among other methods due to its cost-effective, highly efficient, easy operation, simple design, and availability of a wide range of adsorbent materials [1, 3, 9]. Adsorption techniques that use biopolymers have recently been made to remove heavy metals and dyes from wastewater.
Among the biopolymers that have attracted the attention of researchers for being developed for water treatment is chitosan (Cs). This was attributed to the advantages that Cs has over the other adsorbents. It is characterized by its biocompatibility, biodegradability, antibacterial, non-toxicity, and possessing adsorption properties [10, 11]. The adsorption properties of Cs were attributed to the presence of amine and hydroxyl groups in its structure, which allowed it to adsorb contaminants from wastewater via complex formation [10,11,12]. Cs has a high effectiveness for anionic dye adsorption over cationic dyes because of its cationic nature. With progress in science and technology, innovative strategies for improving Cs adsorption performance towards cationic dyes have emerged [13,14,15]. Additionally, pH sensitivity and swelling susceptibility limit the practical use of pure chitosan. In order to resolve these problems, Cs was modified with other organic and/or inorganic materials that have affinity for cationic dyes such as graphene oxide, manganese dioxide, sodium alginate, clay, magnetic nanoparticles, silica, carbon materials, cellulose, and karaya gum [10, 13, 16,17,18,19,20,21,22]. Furthermore, these materials provide Cs with good thermal and chemical stability [10, 23].
Clay minerals, for example, have been used to make CS composites due to their environmentally friendly and inexpensive manufacturing methods, as well as their promising physical and chemical properties. Talc (T) is a type of natural clay with the chemical formula Mg3Si4O10(OH)2. It is composed of three layers: a monolayer of magnesium hydroxide (MgO⋅H2O) sandwiched between two layers of silicate (SiO2) [24, 25]. Layered talc particles are hydrophobic on their basal planes due to the presence of siloxane groups (–Si–O–Si–) but edge hydroxyl groups (–SiOH & –MgOH) are hydrophilic [26]. It was characterized by its abundance, low cost, high surface area, low electrical conductivity, good thermal stability, and chemical inertness [27, 28]. Owing to all these characteristics, T was used in numerous industrial applications, including water treatment, paper, plastic, rubber, composites, paper, cosmetics, and medicines [28, 29]. Recently, Abil and Arefinia [25] studied the effect of talc particles on the ability of a polyurethane coating to protect carbon steel from corrosion. Kalantari et al. [30] integrated various amounts of talc into polysulfone polymer, with the goal of separating carbon dioxide from methane. T has been utilized as an adsorbent in water treatment to remove contaminants from water, either alone or in conjunction with other inorganic/organic materials [24, 26,27,28,29, 31,32,33,34,35]. According to the research findings, T has high adsorption affinity for cationic dyes and positively charged metal ions because its structure contains a large number of functional groups such as Mg − O, − OH, Si − O − Si, and O-Si–O linkages [35]. Sprynskyy et al. [26] used talc to eliminate hexavalent uranium. Wenlei et al. [36] presented the adsorption of methyl orange dye on the surface of 50% (w/w) HNO3 acid modified talc. In another study, talc was modified with microwave and acetic acid to adsorb methylene blue (MB) dye [37]. Alizadeh et al. [28] developed Fe3O4@SiO2-IL/Talc nanocomposite for sorption of MB dye. FeSx/talc composite was prepared for the removal of 2,4,6-trichlorophenol under microwave [38]. There are few reports for the synthesis of chitosan talc (CsT) composite. For example Lima et al. [39] prepared CsT using glutaraldehyde as a crosslinker for drug delivery systems. Chitosan/polyvinyl alcohol/talc composite was synthesized to remove anionic dyes (Congo red & Methyl orange) and heavy metals (Pb2+&Cr6+) [24]. They reported that to prevent swelling during the experiment, talc was added to the composite as reinforcement.
Cloisite 30B is composed of a naturally occurring montmorillonite clay [(Ca,Na)0.3Al2(Si,Al)4O10(OH)2xH2O] that has been modified with the organic surfactant methyl tallow bis(2-hydroxyethyl) quaternary ammonium salt [6]. It made up of tetrahedral silicate sheets sandwiched between octahedral alumina sheets. This type of organoclay is of interest as it can provide the adsorbent with additional positive charge arising from the presence of the quaternary ammonium salt surfactant in its composition [40, 41]. At the same time the organic modifier in Cloisite 30B is a bi-functional amphiphilic surfactant (ammonium bearing alkyl chains and hydroxyl groups) [6]. Chitosan/polylactide composite was sensitized with different wt% of Cloisite 30B to control release of the anticancer drug [42]. Recently, Safarzadeh et al. [4] used Cloisite 30B to improve of the adsorption properties of poly(methacrylic acid-co-acrylamide) hydrogel towards MB dye. Recently, Sujithra and Arthanareeswaran modified Cloisite 30 B with PEO after that polyether sulfone/f-(PEO-Cloisite) nanocomposite membranes were prepared for the removal of dyes and salts from waste water [43]. Literature search has revealed that, there are limited studies on synthesis of chitosan/Cloisite 30B based composites for dye removal. Vanaamudan and Sudhakar [6] prepared chitosan/Cloisite 30B composite for sorption of anionic dyes, reactive blue-21 and reactive red-141. Also, chitosan/Cloisite 30B composite was used for the adsorption of Rhodamine-6G [5]. Daraei et al. [44] prepared chitosan/Cloisite 30B nanocomposites with different quantities of Cloisite 30B weight percentage (0, 0.5, 1, and 2%) to be coated over the surface of commercial polyvinylidene fluoride (PVDF) to be used as microfiltration membrane for dye removal.
To our knowledge, there has been no report of the synthesis of ternary composites consists of chitosan (Cs), talc (T), and Cloisite 30B organoclay (C) as will be presented here neither for dye adsorption nor for any applications. In this work, Cs was firstly modified with talc for improving its thermal stability. After that the obtained CsT composite was sensitized different amounts of C. The prepared Cs, CsT composite, and CsTC hybrid composites were characterized by XRD, FTIR, SEM, TGA, surface area, and zeta potential. It is expected that incorporating C into the CsT composite would impart the resulting composite with additional positive charges besides those of Cs and this in turn could further enhance the adsorption performance of the resulting composite towards anionic dyes as well. In a single dye solution, the removal efficiency of the developed CsTC composites in comparison with pure Cs and CsT composite was evaluated using reactive yellow 145 (RY) dye (an anionic dye) and crystal violet (CV) dye (a cationic dye). According to the presented findings, CsTC composites exhibited simultaneous removal of anionic and cationic dyes in a mixed dye solution. Also the ability of CsTC composites for simultaneous removal for both dyes was presented. In addition, the effect of C mass ratio, initial dye concentration, contact time, adsorbent dosage, and pH on the adsorption performance was evaluated. Kinetic and isotherm studies for adsorption of CV and RY dyes using CsTC composites were also studied. Finally, the adsorption mechanism for CV and RY dyes was proposed based on FTIR analysis.
Experimental
Materials
High molecular weight chitosan (Cs), glacial acetic acid, sodium hydroxide, crystal violet (CV), and reactive yellow 145 (RY) were obtained from Sigma-Aldrich. Some properties of CV and RY are listed in Table 1.Talc powder (T) was purchased from Five Fives company, Egypt. Cloisite 30B (C) was bought from Southern Clay Products Inc.
Methods
Chitosan/talc/Cloisite 30B (CsTC) composites, which contain different weight ratios of Cloisite 30B (C) and fixed amounts of chitosan (Cs) and talc (T), were prepared via a simple solution mixing method. The mass ratio of Cs, T, and C was 1:0:0, 1:2:0; 1:2:1, and 1:2:2 to obtain Cs, CsT, CsTC1, and CsTC2 composites, respectively. 1 g of Cs was dissolved in 50 ml of 1% (v/v) acetic acid to get solution A. In another beaker T and C was dispersed in 5 ml distilled water to form solution B. Then, solution B was added to solution A slowly under vigorous stirring for 2 h. Finally, 100 ml of NaOH (2 M) was slowly added to the resulting mixture solutions to separate the obtained white precipitates, which were left in NaOH solutions overnight. After that, they were filtrated, washed with distilled H2O until pH became neutral, and oven dried at 60 °C for 24 h. For comparison, pure Cs was prepared by the same procedure except the step of adding T and C dispersion.
Characterization
The microstructure of the various samples, including Cs, CsT, CsTC1, and CsTC2 composites was detected by scanning electron microscopy (SEM, Quanta FEG-250). The X-ray analysis (XRD, Panalytical X’Pert PRO) measurement was used to identify the crystal structure. The functional groups of the prepared materials were detected by fourier transform infrared spectrometry (FT-IR, 8400 Shimadzu, Japan) in the range of 4000–400 cm−1. The thermal stability was evaluated by thermogravimetric analysis (TGA-50Shimadzu) that measured under nitrogen atmosphere from 25 to 700 °C at 10 °C/min heating rate. The value of specific surface area was estimated by measuring N2 adsorption–desorption isotherm using Quanta chrome apparatus and pore size distribution was determined using the DFT method. Zeta potential analysis was carried out using Nano-ZS (Malvern Instruments Ltd., UK).
Adsorption Experiments
In a batch mode reactor, crystal violet (CV) and reactive yellow 145 (RY) were chosen as examples of cationic and anionic dyes, respectively, to study the adsorption properties of the prepared samples. The experiments for CV or RY adsorption were conducted by adding the adsorbent to dye solution under constant stirring and every 30 min, 3 ml of this dispersion was taken out and centrifuged at 5000 rpm for 2 min. The remaining concentration of CV and RY dye was estimated by the absorbance of the major peak at 586 nm and 419 nm, respectively. All experiments were performed twice, with the average results presented. The following equations are used to calculate the dye removal percentage (R%, Eq. 1) and adsorption capacity (qt, Eq. 2) for all samples.
where \({\mathrm{C}}_{0}\) is the initial dye concentration and \({\mathrm{C}}_{t}\) is the dye concentration at a certain time (t).The volume of the dye solution is V, and the sample weight is w. For the samples that exhibited the highest dye removal efficiency, the effect of varying contact duration, adsorbent does, pH, and initial dye concentration was investigated in a separate dye solution.
The kinetics of dye adsorption behavior on selected adsorbent was investigated using the pseudo-first order (PFO, Eq. 3), pseudo-second order (PSO, Eq. 4), intra-particle diffusion (IPD, Eq. 6), and Elovich (Eq. 7) models [45,46,47,48]. From the PSO model, h (mg/g · min) as the initial adsorption rate can be determined as expressed by Eq. 5.
PFO
PSO
IPD
Elovich
Where K1, K2, and Ki are the rate constant of PFO, PSO, and IPD model, respectively. C is the intercept of IPD model and β (mg/g.min) is desorption constant.
To further understand the mechanics of the adsorption process, the Langmuir (Eq. 8) [49], Freundlich (Eq. 10) [50], Temkin (Eq. 11) [51], and Dubinin-Radushkevich (Eq. 12) [52] isothermal models were used to describe the sorption data at equilibrium.
Langmuir isotherm
Freundlich isotherm
Temkin isotherm
Dubinin–Radushkevich isotherm
Where Ce is the dye concentration at equilibrium and its corresponding adsorption amount is qe. KL, KF, and bT are the Langmiur, Freundlich, and Temkin isotherm constants, respectively. Equation 9 is used to estimate the separation factor, RL, and n corresponds to the intensity parameter in Freundlich model. A is related to the equilibrium binding constant. R and T are the universal gas constant and working temperature, respectively. The Polanyi sorption potential is \(\varepsilon\), which determined using Eq. 13. Eads is the adsorption free energy that is estimated by using Eq. 14.
Finally, the activity of the prepared composites (CsTC1 and CsTC2) for simultaneous removal of both cationic crystal violet (CV) and anionic reactive yellow 145 (RY) dye were investigated in a mixed dye solution.
Results and Discussion
Characterization Studies
XRD Analysis
The XRD technique was used to study the crystalline structure. Figure 1 displays the XRD patterns of pristine Cloisite 30B (C) as well as CsT composite before and after inclusion of C. XRD pattern of pure cloisite 30B, Fig. 1a, exhibits a peak at 2θ = 4.67° [5, 6, 53]. For CsT composite, Fig. 1b, the peaks related to T were observed at 2θ = 9.34°, 18.83°, 28.5°, 35.12°, and 48.42° while the peak related to Cs was observed at 19.29° [24, 27]. Bragg's formula, Eq. (15), was used to estimate the distance between the talc sheets (d) in different adsorbents based on the XRD patterns [28].
where n, λ, and θ denote a positive integer, the incident wave's wavelength, and the Bragg angle of the diffraction peak, respectively. As depicted in Fig. 1 (B), the d-spacing of talc in CsT at 2θ = 9.34 degrees was 0.95 nm, and it dropped gradually to 0.94 nm at 2θ = 9.41 degrees due to the inclusion of Cloisite 30B clay particles on the surface of talc layers.
Comparing the XRD pattern of CsT composite with that of CsTC hybrid composites, it was found that a new peak related to Cloisite 30B (C) had developed at 4.84° and 4.94° for CsTC1 (Fig. 1c) and CsTC2 (Fig. 1d), respectively [6]. Additionally, it was noticed that this peak become broader and its intensity was reduced with increasing loading of clay as shown in Fig. 1 (B) in the range of 2θ = 4–11 indicating intercalation between polymer and clay. The inter layer basal spacing at 4.67° was 1.89 nm for pure Cloisite 30B clay which agree with literature [54]. This value was decreased to 1.82 nm for CsTC1 and 1.78 nm for CsTC2. This result may be due to layer compression of clay. Also, Daraei et al [44] found that by raising the amount of Cloisite 15A, the characteristic peak shifted to a larger angle due to a decrease in interlayer spacing. Furthermore, the intensities of the diffraction peaks of talc were dramatically reduced in the XRD pattern of composites when compared to that of CsT. The crystalline peak of T was shifted to higher degree, 9.37° for CsTC1 and 9.41° for CsTC2. Therefore, these results indicate a considerable interaction between chitosan, talc, and Cloisite 30B [24].
Using the Debye Scherrer equation, XRD revealed that the particle size of C, CsTC1, and CsTC2 was 5.57, 3.34, and 3.07 nm, respectively. The decrease in the particle size of Cloisite 30B clay after incorporation in polymer matrix agrees with previous reports [5, 6].
SEM Analysis
The surface morphology of all the prepared samples such as Cs, CsT, CsTC1, and CsTC2 composites was studied by SEM analysis as shown in Fig. 2. Pure chitosan (Cs) is free of pores and it has relatively homogenous and smooth surface, Fig. 2a. From SEM figure of CsT composite, Fig. 2b, it was observed that there was a good dispersion of T within the Cs matrix. This might be due to the good compatibility and interaction between OH groups on the surface of the talc and OH and/or NH2 groups on Cs, which resulted in a good adhesion between them. It was also observed that the addition of T significantly increased the surface roughness relative to unmodified Cs. For the CsTC1 and CsTC2 hybrid composites in Fig. 2c and d, respectively, there was a dramatic change in the microstructure after the incorporation of clay material. The composites exhibited more cracks and voids as well as a rise in the surface roughness relative to pure Cs and CsT composite. The nanocomposite’s surface roughness may be an indication that silicate platelets have been incorporated into the polymer matrix. In addition, this rough surface indicates higher surface area as will illustrated later in BET section. Consequently, this can improve the composite's ability to adsorb dyes on its surface. However, the surface of CsTC composites, even with high loading of clay, is free of any agglomeration demonstrating uniform distribution of clay particles within the chitosan matrix. This could be due to the presence of hydroxyl groups in Cloisite 30B, which gives it a hydrophilic character, facilitates better interaction between clay particles and chitosan.
FTIR Analysis
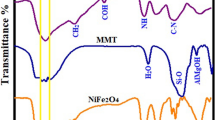
The FTIR spectroscopy was used to analyze the interaction between composite constituents, including Cs, T and C. Figure 3 shows the FTIR spectra of CsTC1 and CsTC2 hybrid composites in addition to pure Cs and CsT composite for comparison. Spectrum in Fig. 3a, shows the characteristic bands of Cs that appeared at 3200 cm−1 (− OH & N–H stretching), 2849& 2926 cm−1(− C − H stretching), 1630 cm−1 (amide stretching), 1533 cm−1 (N − H bending), 1426 cm−1(− C − H symmetrical deformation), and 1063& 1020 cm−1(C − O stretching) [13, 55, 56]. For CsT composite, Fig. 3b, the new absorption bands at 3673, 996, 660 and 460 cm−1 were related to the functional groups of T. The band at 3673 cm−1was attributed to the stretching vibration of Mg − OH. The band at 660 cm−1 was ascribed to Si − O − Mg bonds. The bands at 460 cm−1and 996 cm−1 were attributed to the stretching vibration of Si − O − Si bonds [26, 28, 57]. In addition, the characteristic bands of Cs were appeared with some alterations for example the intensity of O − H stretching, − NH stretching, and C − O stretching bands of Cs increased and there was a slight shift. The composite formation may take place as a result of interactions between OH groups on the surface of the talc and OH and/or NH2 groups on Cs. The bands of amide and amine of Cs that appeared at 1630 and 1533 cm−1 in Cs spectrum were shifted to 1658 and 1550 cm−1. This shift was attributed to the presence of an electrostatic interaction between the negatively charged sites of T and amine groups of Cs [58]. The spectra of CsTC hybrid composites (Fig. 3 c & d) exhibited the absorption bands of Cs, T, as well as C. The band at about 3670 cm−1may be related to Al − O − H and Si − O − H stretching vibration of Cloisite 30B [44, 59]. The clear shift and broadening of the chitosan peaks in the range of 1500 − 1650 cm−1 verifies the interaction of the protonated amine groups in chitosan with oxygen species of Si − O& Al − O in Cloisite 30B clay [44]. The strong band between 1190 and 850 cm−1 is made up of the overlapping bands of the talc, Cs, and C, as well as the new Si–O–C and C–NH–C vibrations. The Al − OH and Si − OH vibrations of Cloisite 30B are appeared at 995 & 795 cm−1, respectively, as clearly shown for high doping CsTC composite [6]. These results verify the inclusion of Cloisite 30B clay in the CsT matrix. As the loading of Cloisite 30B clay increases, it can be seen that the intensities of the peaks become stronger, suggesting more loading of Cloisite 30B.
TGA Analysis
Thermogravimetric (TGA) analysis was used to evaluate the thermal stability of samples (Cs, CsT, CsTC1, and CsTC2) as shown in Fig. 4. In this test, the weight loss percentage was determined as a function of temperature in N2 atmosphere. The thermal degradation of Cs occurred in three stages, as can be seen in Fig. 4a. The first stage occurred approximately at 100 °C as a result of moisture evaporation. The second stage observed at around 220 °C and could be attributed to the degradation of Cs chains. The third stage was noticed in the range of 365–531 °C due to the decomposition of pyranose rings of Cs with ring-opening reaction [10]. It is noticed that modification of Cs with T significantly enhanced its thermal stability as revealed in Fig. 4b. At 700 °C the weight loss was 45.5% for Cs and 14.4% for CsT. This enhancement in the thermal stability of the prepared CsT relative to pure Cs could be attributed to the interaction via hydrogen bonding between Cs and hydroxyl groups of talc as well as the high thermal stability of T [24, 60, 61].The prepared composites CsTC1 (Fig. 4c) and CsTC2 (Fig. 4d) exhibited a higher thermal stability than the unmodified Cs. This demonstrates that the incorporation of Closite 30B improves the thermal stability of polymer matrix in accordance with previous results [4, 6]. The hybrid composites showed approximately the same weight loss and there were no significant differences in the weight loss curves. The small reduction in thermal stability is most likely due to the creation of Cloisite 30B clay platelet agglomerates and phase separation at higher concentrations of Cloisite 30B, which promotes local stress generation and weakens the interaction bonds between CsT and Cloisite 30B particles. Poddar et al. [62] have proposed that a lower amount of Cloisite 30B clay improves thermal stability, whereas, higher clay concentrations seem to reduce thermal stability. Furthermore, it was noticed that with incorporating C into CsT composite, a decrease in the degradation temperature of the resulting composites (CsTC1 and CsTC2) occurred. This was ascribed to the low thermal stability of the organic modifier (surfactant) in the C composition. In conclusion, the weight loss in case of composites (20–22%) was very lower than that of Cs (45.5%) at 700 °C. Table 2 provides the mass loss for all samples at 700 °C. This demonstrated that the thermal stability of CsTC composites was improved after the incorporation of Cloisite 30B, as recently reported by Safarzadeh et al. [4].
Zeta potential Analysis
To explore the surface charge of the prepared samples (Cs, CsT, CsTC1, and CsTC2 composites), the zeta potential (ZP) was determined as revealed in Fig. 5. Zeta potential value of pure Cs was − 9.98 mV. The addition of talc to Cs increased the negative charges on the surface and the ZP value increased to − 11.4 mV. This could be due to the negative charge on the talc's surface. As a result of the results discussed above, it is predicted that CsT composite may show higher adsorption affinity towards cationic dyes (CV) and on the otherwise lower adsorption affinity towards anionic dyes (RY) in comparison with pure Cs, as will be confirmed in the dye adsorption section. The inclusion of Cloisite 30B clay to CsT composite decreased the value of the ZP to − 10.9 mV and − 5.95 mV for CsTC1 and CsTC2 composites, respectively. Similar observations in zeta potential values have also been reported for PMMA/Cloisite 30B composites containing varying concentrations of cloisite 30B clay [62]. They found that increasing clay content decreased the zeta potential to less native values. Bhanvase et al. [63] observed a consistent trend of zeta potential drop with nanocomposite formation and increasing clay concentration. This decrease suggests an increase in the latex stability due to the uniform distribution of silicate layers in the polymer matrix [62].
Surface Area Analysis
Figure 6 A reveals the N2 adsorption–desorption isotherms of various samples including Cs, CsT, CsTC1, and CsTC2 composites. It is clear that the isotherms of the formed composites (CsTC1 and CsTC2) after the incorporation of Cloisite 30B clay exhibited a hysteresis loop that displaying their mesoporous character. The type of the isotherm is type IV. The specific surface area value was 13.53 m2/g for Cs and after combination with talc it was increased to 14.60 m2/g. For CsTC1 and CsTC2 composites, the surface area reached 15.16 and 17.65 m2/g, respectively.
Figure 6 B shows the estimated pore size distribution using DFT model for all samples. It is noticed that the addition of Cloisite 30B clay resulted in the development of larger pores. The size of the pores is less than 50 nm that confirmed the mesoporous properties. The average pore size was 2.81 nm for Cs, 5.32 nm for CsT, 7.86 nm for CsTC1, and 9.62 for CsTC2 composite. In addition, the total pore volume was 0.019, 0.039, 0.060, and 0.085 cc/g for Cs, CsT, CsTC1, and CsTC2, respectively. Table 2 lists the values the textural parameters for all samples. It is concluded that the first modification for Cs by talc followed by a second modification using Cloisite 30B results in a stepwise enhancement for the textural parameters, including surface area, average pore size, and total pore volume, in addition to the development of mesoporous character. These results predicted the improved dye adsorption properties of the prepared composites as previously reported [48].
Dye Adsorption Studies
Single System Dye Removal
Dye removal efficiency of the developed chitosan/talc/Cloisite 30B hybrid composites (CsTC1 & CsTC2) in comparison with pure chitosan (Cs) and chitosan/talc composite (CsT) towards cationic dye and anionic dye was evaluated by examining the adsorption performance of the prepared samples in the CV and RY dye solutions separately. Figure 7 shows the UV–Vis spectra of cationic CV dye solution (C0 = 30 mg/L) at 0 min and after 240 min of adsorption time using several adsorbents. These spectra indicate that after 240 min, both pure Cs (Fig. 7a) and CsT (Fig. 7b) caused a small decrease in the main absorption band of CV at 586 nm. However, once C was incorporated to get CsTC1, there was a significant decline in the intensity of the CV dye, Fig. 7c. Increasing concentrations of clay resulted in a faster decline in the CV peak. For the CsTC2 (Fig. 7d) the peak of CV dye was almost diminished, indicating the highest adsorption affinity for CV dye. The removal percentage for CV dye using Cs, CsT, CsTC1, and CsTC2 adsorbents was estimated as shown in Fig. 7e. Cs had the lowest removal efficiency of only 20%. The addition of T to give CsT composite, caused a small increase in the removal efficiency to 22.7%. On the other hand, the modification with C significantly increased the removal efficiency up to 68.8% and 96.5% for CsTC1 and CsTC2, respectively. These results might be explained based on the charge of Cs, T, and C in addition to the dye molecules. Because of its cationic nature, chitosan exhibited low adsorbing activity towards cationic dyes (CV). Thanks to the hydrophilic O–H groups on the backbone of talc thus it is expected that cationic dye molecules can be adsorbed onto talc via electrostatic interactions [26, 28]. But here, it was noticed that the adsorption activity of CsT towards cationic dye wasn't so high. This was attributed to that the Cs has a positively charged surface owing to the presence of protonated amine in its structure. Hence, when T was incorporated into Cs matrix, there might be an electrostatic interaction between the positively charged surface of Cs and negatively charged surface of T [64]. This might lead to suppression in the adsorption activity of CsT composite for cationic CV dye. However, with the incorporation of C, the adsorption activity of the resulting composites (CsTC1 and CsTC2) towards cationic CV dye was enhanced with increasing clay content. This may be owing to that Cloisite 30B clay has negative active sites (Si–O– and OH– groups) that can interact with dye molecules via forming hydrogen bonds. Reddy et al. [65] reported that Cloisite 30B has a net negative charge on the surface. This in accordance with the obtained dye removal efficiency results, so as clay content in the composite increased as the adsorption activity of CsTC composite increased.
Based on these results, CsTC2 composite was selected to investigate its kinetics and isotherms for CV dye adsorption as it exhibited the highest adsorption activity towards the CV dye. Additionally, the influence of pH solution, adsorbent dose, contact time and initial concentration of dyes on the removal of CV by CsTC2 composite was investigated.
The activity of samples including, Cs, CsT, CsTC1, and CsTC2 towards adsorption of anionic reactive yellow 145 dye (RY, C0 = 35 mg/L) was also studied. Figure 8 presents the time dependent UV–vis spectra of RY dye solution in the presence of several adsorbents. The maximum absorption band of RY dye at 419 nm gradually decreased with increasing adsorption time. It can be seen that the CsTC1 sample, Fig. 8c, exhibited the highest reduction of the anionic dye concentration. The removal percentage for RY dye using Cs, CsT, CsTC1, and CsTC2 adsorbents was estimated as shown in Fig. 8e. Cs showed 81.4% removal efficiency. After the addition of T to form CsT composite, the removal for RY decreased to 69.3%. On the other hand, it was observed that with the inclusion of C, the removal efficiency showed an increase up to 98.4% for CsTC1. A further increase in the amount of C caused a decrease in the RY removal efficiency to 84.7%. The charge of the Cs, T, and C, as well as the dye molecules, could account for these findings. Cs has positive charges, so it has high adsorption activity towards the anionic RY dye and this was confirmed by the obtained result in Fig. 8a and e. However, it was found that when T was incorporated into the Cs matrix, the affinity of the resulting composite was reduced. To clarify the reason for this finding, the adsorption of raw talc was studied and it was found that pure talc powder has no any adsorption ability for RY (not shown) due to repulsion between negative function groups of talc and anionic dye molecules. Thus, it could be speculated that the addition of talc to Cs led to a clear decrease towards the RY removal performance when using the obtained CsT (Fig. 8 b and e) in comparison with unmodified Cs. On the other hand, it was observed that with the inclusion of C, the adsorption activity of the resulting composite (CsTC1) towards anionic (RY) dye significantly increased. This might be explained as follows the organic modifier in cloisite30B is a bifunctional amphiphilic surfactant (ammonium bearing alkyl chains and hydroxyl groups) [6]. Thus, the presence of ammonium cations in its structure would provide strong electrostatic interaction between C and the anionic dye molecules, which resulted in a high percentage removal for RY (Fig. 8c and e). But with a further increase in the amount of C, a decrease in the removal of the dye was observed (Fig. 8d and e). This could be explained by the tendency of clay to form agglomerates in the polymer matrix when used in a high amount. Clay particles that are aggregated may not absorb dye molecules as effectively as those that are scattered. The presence of such agglomerates might have an influence in blocking the effect of ammonium groups on the adsorption of anionic dye, as shown by the decrease in RY removal by CsTC composite with high clay content. The same phenomenium was observed by Safarzadeh et al. [4]. They found that high concentration of Cloisite 30B in the poly(methacrylic acid-co-acrylamide) decreased the adsorption efficiency for MB dye, owing to reducing the penetration of MB-dye molecules into the nanoclay composite adsorbent structure. But this assumption isn’t supported by SEM photos. Therefore, we suggest another explanation for these results. In comparison with CsTC2, CsTC1 composite contains higher amounts of Cs, which has high adsorption capacity for anionic RY dye. As a result of the synergistic effect of Cs and C in the CsTC1 composite, the removal efficiency of the produced CsTC1 composite towards anionic dye was greatly improved. Whereas, increasing clay content in CsTC2 composite made the adsorption characteristics of clay more effective. Pure chitosan (Cs) exhibited good adsorption properties towards RY dye (81.4%, Fig. 8e) whereas pure Cloisite 30B (C) showed intermediate adsorption ability for RY dye under identical conditions (62.5%, data not shown). This means that Cs is substituted with less active component, consequently the activity of CsTC2 composite towards adsorption of anionic RY dye was decreased.
Owing to the highest adsorption affinity of CsTC1 towards anionic RY dye, the kinetics and isotherms for RY adsorption onto CsTC1 were also investigated. Additionally, the influence of contact time, adsorbent dosage, pH, and initial concentration of dyes on the removal of RY by CsTC1 composite was investigated. In conclusion, the obtained results revealed that Cs, T, and C had synergistic effects on the removal efficiency for CV and RY.
Binary System Dye Removal
The activity of CsTC2 and CsTC1 composites towards simultaneous removal of CV and RY was studied. Figure 9a and b shows the absorption spectra of RY-CV mixed solution as a function of time in the presence of CsTC1 and CsTC2, respectively. These results indicated that the developed composites effectively adsorb both cationic and anionic dyes giving our manufactured composites an additional advantage. Pure Cloisite 30B clay (not shown) exhibited adsorption properties for cationic CV dye (100% removal), due to hydroxyl function groups in its structure, and also its adsorption ability for anionic RY dye may be owing to its ammonium cations (62.5% removal). Thus, the incorporation of clay (C) into CsT composite increases the adsorption properties towards CV and RY dyes. Finally, the developed CsTC hybrid composites acquired advantages relative to its constituent parts.
Influence of Solution pH
The pH could have an effect on the surface charge and functional groups ofthe adsorbent material, which would have a direct impact on the overall adsorption capacity. In order to determine the optimal pH, CV removal by CsTC2 composite and RY removal by CsTC1 was performed in the pH range of 4 to 10. NaOH and HCl were used to change the pH of dye solutions. Figure 10a demonstrates a clear improvement in CV removal as pH increases. Because of the low pKa value, CV is almost completely ionized under all conditions and exists as positively charged dye ions. At lower pH, the high concentration of hydrogen ions in the solution would compete with CV+ for the adsorbent's insufficient active sites. As a result, CV removal was reduced in an acidic environment [8]. Also, Cloisite 30B particles become protonated in acidic conditions, resulting in a greater accumulation of positive charge [44], which decreases CV adsorption. At high pH values, the hydroxyl and amido groups in composites would release H+, resulting in an increase in negative charge. The improved electronegative composite would speed the absorption and combination of additional electropositive CV molecules. The data shown in Fig. 10a indicate that the CV removal efficiency increased significantly by increasing initial pH of dye solution from 4 to 8.5. After that increasing pH from 8.5 to 10 had no significant impact on the amount of CV that could be adsorbed. Similar behavior was reported by Kyi et al. [66]. These findings prompted the decision to conduct further tests at a pH of 8.5, which is the pH at which CV solution exists in nature. What’s more, this result is relevant to the implementation of the adsorption process on large scale because it eliminates the requirement to alter the pH of the effluent prior to treatment.
For RY adsorption on CsTC1 composite, Fig. 10b, when the pH goes up, the amount of dye that sticks to composite surface goes down. The dye removal efficiency is almost the same in pH ranged from 4 to 8. At pH 10 the adsorption significantly decreased. This decrease may be caused by the repulsion force between the negatively charged dye molecule and the negative surface of composite. Excess hydroxyl ions decreased protonation of amine groups of chitosan and also reduced the positive charge impact of modifying surfactants included in the Cloisite 30B structure, consequently poor dye removal for anionic RY. As previously mentioned [67], the adsorption efficiency for RY 145 is decreased by increasing pH. Thus, for the rest of our studies on RY adsorption, we chose to start with a solution with a pH of 8.
Influence of Composite Dosage
Cost-effective color removal from wastewater has stimulated research into the smallest effective dose of adsorbent for maximizing dye removal. Various amounts of CsTC2 varied from 0.5 to 1.25 g/L and CsTC1 ranged from 0.1 to 0.8 g/L for removal of CV and RY, respectively. As indicated in Fig. 11, the dye elimination was improved with increased composite dose. This was due to an increase in valid adsorption sites [48]. Adsorption might be sped up at higher doses since there would be additional adsorption sites to interact and combine dye with the composite, leading to faster removal [48]. Testing showed that CsTC2 dosage of 1 g/L and CsTC1 dosage of 0.57 g/L was effective in removing CV and RY, respectively. Therefore, these doses were employed in the subsequent testing.
Influence of Contact Time
The influence of contact time on the removal of RY dye (40 mg/L) using CsTC1 and CV dye (30 mg/L) using CsTC2 was studied and the obtained results are presented in Fig. 12.From this figure, it is indicated that whatever the investigated adsorbent material (CsTC1 or CsTC2) and adsorbate molecules (CV or RY dye), by increasing the contact duration from 30 to 240 min, the removal percentage for both dyes increased until the adsorption rate achieved equilibrium state. This was owing to the natural adsorption process. Because all adsorbing sites are free, the dye molecules can interact with them, and the amount of residual dye molecules drops dramatically, causing higher removal efficiency. Afterwards, as the adsorption sites were saturated with pollutant molecules, the removal efficiency becomes almost constant at 270 min [68]. Similar findings were observed in other studies [69, 70]. The optimal adsorption time was 240 min.
Influence of Initial Concentration of Dyes
In these experiments, the impact of varying the concentration of CV dye (10, 15, 20, 25, 30, 35, and 40 mg/L) on the removal percentage was studied using CsTC2 as adsorbent sample (Fig. 12). In the case of RY dye, the initial dye concentrations were (30, 35, 40, 50, and 60 mg/L) and the respective adsorption percentage onto CsTC1 was estimated, as shown in Fig. 12. Both adsorbents, CsTC1 and CsTC2, exhibited the same trend, that is, as the concentration of adsorbate dye (CV or RY) increased, the percentage of dye removal reduced. This was owing to the fact that raising the dye concentration increases the number of dye molecules, but the number of active adsorption sites on the adsorbent material is fixed, hence increasing the initial dye concentration decreases the decolorization effectiveness. Other studies reported similar results [48, 70]. The optimal concentration for CV and RY dyes was 30 mg/L and 40 mg/L, respectively.
Adsorption Kinetics
CsTC2 composite was the best adsorbent for CV dye, and CsTC1composite was the best adsorbent for RY dye. Therefore, the adsorption kinetics of CV dye was examined onto CsTC2 sample, and the adsorption kinetics of RY dye was examined onto CsTC1 sample. Figure. 13 shows the linear fitting of PFO, PSO, IPD, and Elovich kinetic models to the experimental adsorption data. For CV, 1 g/L of CsTC2 was added to CV solution with initial concentration of 30 mg/L. The adsorbent dose was 0.57 g/L for CsTC1 using RY solution with initial concentration of 40 mg/L. The value of the correlation coefficient (R2) is used to evaluate the validity of each kinetic model.
Table 3 summarizes the kinetics calculation results and the correlation coefficient (R2) values for PFO, PSO, IPD, and Elovich model for CV and RY adsorption onto CsTC2 and CsTC1, respectively. Owing to the extraordinarily high correlation value, 0.994 for CV and 0.997 for RY, the PSO kinetic model was ideal for the adsorption processes. This demonstrated that the CV adsorption onto CsTC2 and RY adsorption onto CsTC1 were controlled by a chemical adsorption step [48, 70].
Adsorption Isotherms
Figure. 14 illustrates the adsorption isotherm models, including Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich for adsorption of CV and RY onto CsTC2 and CsTC1, respectively. The sorption equilibrium studies were carried out using CV with initial concentrations of 10, 15, 20, 25, 30, 35, and 40 mg/L using CsTC2 composite. For RY, the initial concentrations were 30, 35, 40, 50, and 60 mg/L using CsTC1 composite. According to the correlation coefficients (R2), we can select the best fitting model.
The correlation coefficient (R2) value and model parameters for each isotherm were estimated as shown in Table 4. From these results, it could be seen that the R2 value of the Langmuir isotherm model was very high (0.992 for CV and 0.992 for RY), indicating it was the most suitable model to describe the adsorption processes. The adsorption capacity (qm) values for CsTC2 and CsTC1 composites were determined using this isotherm model and were 37.03 mg/g for CV and 76.9 mg/g for RY, respectively. It was reported that the maximal adsorption capacity of Fe3O4/Cs was only 46.40 mg/g for RY dye [71]. The sorption process was unfavorable at RL > 1, favorable at 0 < RL < 1, and irreversible at RL = 0 (37, 55). The value of the separation factor (RL), was equal to 0.012 for CV (C0 = 30 mg/L) and 0.005 for RY (C0 = 40 mg/L). The obtained results demonstrated that the adsorption of CV and RY on CsTC2 and CsTC1composites, respectively, were desirable.
FTIR After Adsorption
FTIR spectra of CsTC1 and CsTC2 composites after the adsorption of RY and CV, respectively, relative to that before dye adsorption are displayed in Fig. 15. It was observed that the adsorption of RY dye on the surface of CsTC1 and also the adsorption of CV on the surface of CsTC2 were accompanied by a small shift of the absorption peaks to higher wavenumber in addition to a clear decrease in their intensities, including the characteristic peaks at 3673 cm−1, 3300 cm−1, strong band between 1190 and 850 cm−1, which are caused by Mg − O − H of T as well as Al − O − H and Si − O − H stretching vibration of Cloisite 30B [26, 28, 44, 57, 59], the − OH and − NH of Cs, and the overlapping bands of the talc, Cs, and C, respectively. These results indicate that these functional groups participated in the dye adsorption process (See Fig. 16)
Proposed Adsorption Mechanism
The proposed mechanism for simultaneous adsorption of CV and RY on the surface of the developed CsTC hybrid composites is based on the structure, function groups, and charge of their constituent components. CsTC composites consist of chitosan (Cs), talc (T), and Cloisite 30B clay. Chitosan contain hydroxyl groups as well as amino groups that make chitosan a cationic polyelectrolyte. Talc is made up of magnesium hydroxide (MgO⋅H2O) layers sandwiched between two silicate (SiO2) layers, and it is hydrophobic on their basal planes due to the presence of siloxane groups (–Si–O–Si–) but hydrophilic at edges due to the presence of hydroxyl groups (–SiOH & –MgOH) [26]. Cloisite 30B is a modified montmorillonite clay, and the organic modifier in Cloisite 30B is a bifunctional amphiphilic surfactant (ammonium bearing alkyl chains and hydroxyl groups) [6]. Based on the FTIR results, CsTC composites possess a large number of functional groups such as − OH, − NH3+, Si − O, Mg − OH, and AL − OH. After dye adsorption, the intensity of the peaks of these groups were significantly decreased suggesting that these functional groups were involved in the dye adsorption. There are different types of interaction forces can be occurred between the dye molecules and adsorbents such as electrostatic attraction and hydrogen bonding. The electrostatic attraction force arose as a result of the difference in charges between the dye molecules and the surface of the adsorbent. This interaction can occur between the molecules of cationic CV dye and negatively charged groups of T present in CsTC [72, 73]. At the same time the molecules of RY dye have negatively charged groups (− SO3−), so strong electrostatic attraction would occur between RY molecules and positively charged groups of Cs (− NH3+) and C (ammonium cations), which are present in CsTC composites. In addition to the electrostatic attraction force, another type of force can be involved in the dye adsorption process is the hydrogen-bonding attraction. Hydrogen-bonding attraction arose as a result of formation of H-bonds between amine groups in the structure of dye molecules and − OH groups present in the structure of the adsorbent material [72, 73]. Thus hydrogen bonds may be formed between hydroxyl group of talc or chitosan or Cloisite 30B and CV or RY dye. All these forces are illustrated in Scheme 1.
Comparison with Other Adsorbents
To evaluate the usefulness of using the prepared hybrid composites to remove both cationic and anionic dyes,the maximum adsorption capacities of the prepared composites were compared with different adsorbents previously reported in the literature for removal of CV and RY dyes, Table 5. From this comparison, it was concluded that the maximum adsorption capacity (qmax) values of the prepared composites in this work were in line with the adsorbents reported in the literature used for the removal of CV or RY dyes. This indicates that the composite prepared in this work is good candidates for the effectively removal of cationic CV and anionic RY dyes from aqueous solution. In addition, no research has been conducted to investigate the adsorption properties of the same adsorbent towards CV and RY dyes as presented here.
Conclusion
Eco-friendly hybrid composites have been successfully developed from chitosan (Cs), talc powder (T) and Cloisite 30B (C) for the first time for simultaneous removal of cationic (CV) and anionic (RY) dyes. The prepared samples were characterized by XRD, SEM, FTIR, TGA, surface area, and zeta potential. The addition T to Cs polymer matrix resulted in higher thermal stability and increased textural parameters of the produced CsT composite. After that Cloisite 30 B clay was incorporated to CsT with weight ratio of 1:2 and 2:2 to obtain CsTC1 and CsTC2 composites, respectively. These composites exhibited higher thermal stability and larger surface area in comparison with unmodified chitosan. XRD and FTIR analyses confirmed the effective interaction between composite constituents in addition their surfaces were free of agglomeration as presented in SEM photos. The adsorption results showed that the Cloisite 30B (C) amount strongly affected the removal efficiency of the prepared composites towards CV and RY removal; as the amount ratio of C increased, the adsorption activity of the resulting composite towards cationic CV increased whereas the adsorption of anionic RY showed a different trend. The composite with a weight ratio of 2:2 exhibited the highest adsorption activity for CV dye with adsorption capacity of 37.03 mg/g, on the other hand the composite with a weight ratio of 2: 1 was the best one for the removal of RY dye with adsorption capacity of 76.9 mg/g. The adsorption process of RY and CV on these composites followed the pseudo second order model. The langmuir isotherm model described the experimental adsorption data better than the Freundlich, Dubinin–Radushkevich, and Temkin isotherm models, indicating a monolayer sorption process for both dyes. The proposed mechanism for simultaneous adsorption of CV and RY was discussed. The prepared composites in this work can be considered as eco-friendly and economical candidates for simultaneous adsorption of different types of dyes from aqueous solution.
References
Hosseini SA, Daneshvar e Asl, S, Vossoughi M, Simchi A, Sadrzadeh M, (2021) Green electrospun membranes based on chitosan/amino-functionalized nanoclay composite fibers for cationic dye removal: synthesis and kinetic studies. ACS Omega 6(16):10816–10827
Jiang X, Sun Y, Liu L, Wang S, Tian X (2014) Adsorption of CI Reactive Blue 19 from aqueous solutions by porous particles of the grafted chitosan. Chem Eng J 235:151–157
Kandil H, Abdelhamid AE, Moghazy RM, Amin A (2022) Functionalized PVA film with good adsorption capacity for anionic dye. Polym Eng Sci 62(1):145–159
Safarzadeh H, Peighambardoust SJ, Mousavi SH, Foroutan R, Mohammadi R, Peighambardoust SH (2022) Adsorption ability evaluation of the poly (methacrylic acid-co-acrylamide)/cloisite 30B nanocomposite hydrogel as a new adsorbent for cationic dye removal. Environ Res 212:113349
Vanamudan A, Pamidimukkala P (2015) Chitosan, nanoclay and chitosan–nanoclay composite as adsorbents for Rhodamine-6G and the resulting optical properties. Int J Biol Macromol 74:127–135
Vanaamudan A, Sudhakar PP (2015) Equilibrium, kinetics and thermodynamic study on adsorption of reactive blue-21 and reactive red-141 by chitosan-organically modified nanoclay (Cloisite 30B) nano-bio composite. J Taiwan Inst Chem Eng 55:145–151
Abd El-Hamid HT, AlProl AE, Hafiz MA (2022) The efficiency of adsorption modelling and Plackett-Burman design for remediation of crystal violet by Sargassum latifolium. Biocatal Agric Biotechnol 44:102459
Ren X, Xiao W, Zhang R, Shang Y, Han R (2015) Adsorption of crystal violet from aqueous solution by chemically modified phoenix tree leaves in batch mode. Desalin Water Treat 53(5):1324–1334
Huang P, Xia D, Kazlauciunas A, Thornton P, Lin L, Menzel R (2019) Dye-mediated interactions in chitosan-based polyelectrolyte/organoclay hybrids for enhanced adsorption of industrial dyes. ACS Appl Mater Interfaces 11(12):11961–11969
Minisy IM, Salahuddin NA, Ayad MM (2019) Chitosan/polyaniline hybrid for the removal of cationic and anionic dyes from aqueous solutions. J Appl Polym 136(6):47056
Pereira FA, Sousa KS, Cavalcanti GR, França DB, Queiroga LN, Santos IM, Fonseca MG, Jaber M (2017) Green biosorbents based on chitosan-montmorillonite beads for anionic dye removal. J Environ Chem Eng 5(4):3309–3318
El taweil AS, El-Monaem EM, Mohy-Eldin MS, Omer AM, (2021) Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci Rep 11(1):1–15
Swathi M, Roy P, Deepthi V, M, RN Sailaja R, (2021) Removal of toxic dyes from industrial waste water using chitosan grafted itaconic acid nanocomposites. Adv Mater Lett 12(6):1
Isaad J, El Achari A (2020) Chitosan-coated nonwoven polyethylene terephthalate material for efficient removal of cationic and anionic dyes from aqueous solution. Int J Environ Anal Chem 4:1–23
Cui J, Wang X, Yu S, Zhong C, Wang N, Meng J (2020) Facile fabrication of chitosan-based adsorbents for effective removal of cationic and anionic dyes from aqueous solutions. Int J Biol Macromol 165:2805–2812
Rajendiran R, Patchaiyappan A, Harisingh S, Balla P, Paari A, Ponnala B, Perupogu V, Lassi U, Seelam PK (2022) Synergistic effects of graphene oxide grafted chitosan & decorated MnO2nanorods composite materials application in efficient removal of toxic industrial dyes. J Water Process Eng 47:102704
Dotto GL, Rodrigues FK, Tanabe EH, Fröhlich R, Bertuol DA, Martins TR, Foletto EL (2016) Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J Environ Chem Eng 4(3):3230–3239
Jumadi J, Kamari A, Rahim NA, Wong ST, Yusoff SN, Ishak S, Abdulrasool MM, Kumaran S (2019) Removal of methylene blue and congo red by magnetic chitosan nanocomposite: characterization and adsorption studies. J Phys 1397(1):012027
Jabli M (2020) Synthesis, characterization, and assessment of cationic and anionic dye adsorption performance of functionalized silica immobilized chitosan bio-polymer. Int J Biol Macromol 153:305–316
Marrakchi F, Hameed BH, Hummadi EH (2020) Mesoporous biohybrid epichlorohydrin crosslinked chitosan/carbon–clay adsorbent for effective cationic and anionic dyes adsorption. Int J Biol Macromol 163:1079–1086
Vega-Negron AL, Alamo-Nole L, Perales-Perez O, Gonzalez-Mederos AM, Jusino-Olivencia C, Roman-Velazquez FR (2018) Simultaneous adsorption of cationic and anionic dyes by chitosan/cellulose beads for wastewaters treatment. Int J Environ Res 12(1):59–65
Ramakrishnan RK, Padil VV, Wacławek S, Černík M, Varma RS (2021) Eco-friendly and economic, adsorptive removal of cationic and anionic dyes by bio-based karaya gum—chitosan sponge. Polymers 13(2):251
Coura JC, Profeti D, Profeti LP (2020) Eco-friendly chitosan/quartzite composite as adsorbent for dye removal. Mater Chem Phys 256:123711
Kalantari K, M. Afifi A, (2018) Novel chitosan/polyvinyl alcohol/talc composite for adsorption of heavy metals and dyes from aqueous solution. Sep Sci Technol 53(16):2527–2535
Abil E, Arefinia R (2022) The influence of talc particles on corrosion protecting properties of polyurethane coating on carbon steel in 3.5% NaCl solution. Prog Org Coat 172:107067
Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B (2011) Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J 171(3):1185–1193
Sani HA, Ahmad MB, Saleh TA (2016) Synthesis of zinc oxide/talc nanocomposite for enhanced lead adsorption from aqueous solutions. RSC Adv 6(110):108819–108827
Alizadeh A, Fakhari M, Safaei Z, Khodeai MM, Repo E, Asadi A (2020) Ionic liquid-decorated Fe3O4@ SiO2 nanocomposite coated on talc sheets: An efficient adsorbent for methylene blue in aqueous solution. Inorg Chem Commun 121:108204
Moradihamedani P, Kalantari K, Abdullah AH, Morad NA (2016) High efficient removal of lead (II) and nickel (II) from aqueous solution by novel polysulfone/Fe3O4–talc nanocomposite mixed matrix membrane. Desalin Water Treat 57(59):28900–28909
Kalantari K, Moradihamedani P, Ibrahim NA, Abdullah AH, Afifi AB (2018) Polysulfone mixed-matrix membrane incorporating talc clay particles for gas separation. Polym Bull 75(8):3723–3738
Wang Y, Lin H, Lin Z, Yuan Y (2018) Application and prospect of talc as heavy metal passivation agent. IOP Conf Series: Mater Sci Eng IOP Publ 392(3):03202
Rahman A, Kishimoto N, Urabe T (2015) Adsorption characteristics of clay adsorbents–sepiolite, kaolin and synthetic talc–for removal of Reactive Yellow 138: 1. Water Environ J 29(3):375–382
Li SF, Yang SC, Zhao SL, Li P, Zhang JH (2015) Microwave and acid modified talc as adsorbents of methylene blue in aqueous solution. J Serbian Chem Soc 80(4):563–574
Lee YC, Choi M, Yang JW, Shin HJ (2015) Removal of malachite green (MG) from aqueous solutions by adsorption, precipitation, and alkaline fading using talc: kinetic, thermodynamic, and column feasibility studies. Desalin Water Treat 56(7):1918–1928
Huong NT, Son NN, Phuong VH, Dung CT, Huong PT, Son LT (2020) Synthesis Fe3O4/Talc nanocomposite by coprecipition-ultrasonication method and advances in hexavalent chromium removal from aqueous solution. Adsorpt Sci Technol 38(9–10):483–501
Wenlei L, Shanlin Z, Shuang C, Jinhui Z, Ping L, Shuangchun Y (2014) Adsorptive characteristics of modified talcum powder in removing methylene blue from wastewater. Chem Speciat Bioav 26(3):167–175
Li SF, Yang SC, Zhao SL, Li P, Zhang JH (2015) Microwave and acid modified talc as adsorbents of methylene blue in aqueous solution. J Serb Chem Soc 80(4):563–574
Ai MQ, Du H, Lv SS, Chen XG, Zhang CQ, Cai W, Qin WL, Ye Y, Tao CH (2021) Effective removal of 2, 4, 6-trichlorophenol by FeSx/talc composite under microwave. J Environ Chem Eng 9(4):105287
Lima LC, Coelho CC, Silva FC, Meneguin AB, Barud HS, Bezerra RD, Viseras C, Osajima JA, Silva-Filho EC (2019) Hybrid systems based on talc and chitosan for controlled drug release. Materials 12(21):3634
Guler UA, Ersan M, Tuncel E, Dügenci F (2016) Mono and simultaneous removal of crystal violet and safranin dyes from aqueous solutions by HDTMA-modified Spirulina sp. Process Saf Environ Prot 99:194–206
Munir M, Nazar MF, Zafar MN, Zubair M, Ashfaq M, Hosseini-Bandegharaei A, Khan SU, Ahmad A (2020) Effective adsorptive removal of methylene blue from water by didodecyldimethylammonium bromide-modified Brown clay. ACS Omega 5(27):16711–16721
Nanda R, Sasmal A, Nayak PL (2011) Preparation and characterization of chitosan–polylactide composites blended with Cloisite 30B for control release of the anticancer drug paclitaxel. Carbohydr Polym 83(2):988–994
Sujithra S, Arthanareeswaran G (2022) A novel asymmetric structured nanocomposite PEO-Cloisite based membrane for salt and dye separation. Mater Lett 14:100141
Daraei P, Madaeni SS, Salehi E, Ghaemi N, Ghari HS, Khadivi MA, Rostami E (2013) Novel thin film composite membrane fabricated by mixed matrix nanoclay/chitosan on PVDF microfiltration support: Preparation, characterization and performance in dye removal. J Membr Sci 436:97–108
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Ventenskapsakademiens Hand 24:1–39
Ho YS, McKay GA (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J sanit Eng Div 89:31–59
Ali H, Ismail AM (2022) Structural, morphological, and enhanced dye sorption properties of mesoporous TiO2/ZnS nanocomposite synthesized by SILAR method. J Inorg Organomet Polym Mater 32(1):326–343
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich H (1906) Uber die adsorption in lo sungen. Z Phys Chem 57:385–471
Tempkin MI, Pyzhev VJ (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327–356
Dubinin M, Radushkevich L (1947) Equation of the characteristic curve of activated charcoal. Chem Zentralbl 1:875
Mahmoudian M, Abdali A, Eskandarabadi SM, Nozad E, Enayati M (2021) The performance of an efficient polymer and Cloisite30B derivatives in the adsorption desulfurization process. Polym Bull 78(2):795–812
Sodeifian G, Nikooamal HR, Yousefi AA (2012) Molecular dynamics study of epoxy/clay nanocomposites: rheology and molecular confinement. J Polym Res 19(6):1–2
Ali H, Ismail AM (2022) Recyclable and biodegradable Ag@ chitosan nanocomposite beads synthesized in one-step for catalytic hydrogenation of 4-nitrophenol. J Polym Environ 6:1–2
Wang X, Tang R, Zhang Y, Yu Z, Qi C (2016) Preparation of a novel chitosan based biopolymer dye and application in wood dyeing. Polymers 8(9):338
Ossman ME, Mansour MS, Fattah MA, Taha N, Kiros Y (2014) Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions. Bulg Chem Commun 46:629–639
Thakur G, Singh A, Singh I (2016) Chitosan-montmorillonite polymer composites: formulation and evaluation of sustained release tablets of aceclofenac. Sci Pharm 84(4):603–617
Sahoo D, Nayak PL (2012) Synthesis and characterization of chitosan/cloisite 30B film for controlled release of ofloxacin. J Appl Polym Sci 123(5):2588–2594
Sahoo G, Sarkar N, Swain SK (2017) Biomass-based nanocomposite for packaging applications. Lignocellulosic fibre and biomass-based composite materials. Woodhead Publishing, Sawston, pp 123–146
Swain SK, Kisku SK, Sahoo G (2014) Preparation of thermal resistant gas barrier chitosan nanobiocomposites. Polym Compos 35(12):2324–2328
Poddar MK, Sharma S, Moholkar VS (2016) Sonochemical synthesis of PMMA/cloisite 30B nanocomposites: a mechanistic investigation. In Macromol Symp 361(1):82–100
Bhanvase BA, Pinjari DV, Gogate PR, Sonawane SH, Pandit AB (2012) Synthesis of exfoliated poly (styrene-co-methyl methacrylate)/montmorillonite nanocomposite using ultrasound assisted in situ emulsion copolymerization. J Chem Eng 181:770–778
Lertsutthiwong P, Noomun K, Khunthon S, Limpanart S (2012) Influence of chitosan characteristics on the properties of biopolymeric chitosan–montmorillonite. Prog Nat Sci 22(5):502–508
Reddy BN, Rauta PR, Venkatalakshimi V, Sreenivasa S (2018) Synthesis and characterization of cloisite-30B clay dispersed poly (acryl amide/sodium alginate)/AgNp hydrogel composites for the study of BSA protein drug delivery and antibacterial activity. Mater Res Express 5(2):025403
Kyi PP, Quansah JO, Lee CG, Moon JK, Park SJ (2020) The removal of crystal violet from textile wastewater using palm kernel shell-derived biochar. Appl Sci 10(7):2251
Benkaddour S, Slimani R, Hiyane H, El Ouahabi I, Hachoumi I, El Antri S, Lazar S (2018) Removal of reactive yellow 145 by adsorption onto treated watermelon seeds: kinetic and isotherm studies. Sustain Chem Pharm 10:16–21
Abdelhamid AE, Kandil H (2022) Facile approach to synthesis super-adsorptive hydrogel based on hyperbranched polymer for water remediation from methylene blue. React Funct Polym 31:105312
Ali H (2020) Ternary system from mesoporous CdS–ZnS modified with polyaniline for removal of cationic and anionic dyes. Res Chem Intermed 46(1):571–592
Ali H, Ismail AM (2021) Developing montmorillonite/PVDF/PEO microporous membranes for removal of malachite green: adsorption, isotherms, and kinetics. J Polym Res 28(11):1–17
Kalkan NA, Aksoy S, Aksoy EA, Hasirci N (2012) Adsorption of reactive yellow 145 onto chitosan coated magnetite nanoparticles. J Appl Polym Sci 124(1):576–584
Mashkoor F, Nasar A, Asiri AM (2018) Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using tectonagrandis sawdust as a very low-cost adsorbent. Sci Rep 8(1):1–6
Lee YC, Kim JY, Shin HJ (2013) Removal of malachite green (MG) from aqueous solutions by adsorption, precipitation, and alkaline fading using talc. Sep Sci Technol 48(7):1093–1101
Satapathy MK, Das P (2014) Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J Environ Chem Eng 2(1):708–714
Gabal MA, Al-Harthy EA, Al Angari YM, Salam MA (2014) MWCNTs decorated with Mn0. 8Zn0. 2Fe2O4 nanoparticles for removal of crystal-violet dye from aqueous solutions. Chem Eng J 255:156–164
An S, Liu X, Yang L, Zhang L (2015) Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: Study in single-and binary-solute systems. Chem Eng Res Des 94:726–735
Kumari HJ, Krishnamoorthy P, Arumugam TK, Radhakrishnan S, Vasudevan D (2017) An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: a novel low cost adsorbent. Int J Biol Macromol 96:324–333
Shirsath SR, Hage AP, Zhou M, Sonawane SH, Ashokkumar M (2011) Ultrasound assisted preparation of nanoclay Bentonite-FeCo nanocomposite hybrid hydrogel: a potential responsive sorbent for removal of organic pollutant from water. Desalination 281:429–437
Khan TA, Khan EA (2015) Removal of basic dyes from aqueous solution by adsorption onto binary iron–manganese oxide coated kaolinite: non-linear isotherm and kinetics modeling. Appl Clay Sci 107:70–77
Li S (2010) Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly (acrylic acid-acrylamide methacrylate) and amylose. Bioresour Technol 101:2197–2202
Gholami M, Vardini MT, Mahdavinia GR (2016) Investigation of the effect of magnetic particles on the crystal violet adsorption onto a novel nanocomposite based on κ-carrageenan-g-poly (methacrylic acid). Carbohydr Polym 136:772–781
Mahdavinia GR, Massoumi B, Jalili K, Kiani G (2012) Effect of sodium montmorillonite nanoclay on the water absorbency and cationic dye removal of carrageenan-based nanocomposite superabsorbents. J Polym Res 19:9947–9959
Karmaker S, Sen T, Saha TK (2015) Adsorption of reactive yellow 145 onto chitosan in aqueous solution: kinetic modeling and thermodynamic analysis. Polym Bull 72(8):1879–1897
Lafta AJ, Halbus AF, Daabool FS, Burhan ZS, Fenoon HA, Hussein FH (2014) Adsorption of reactive yellow-145 dye on activated carbons. Int J Chem 3:179–183
Saravanan A, Karishma S, Jeevanantham S, Jeyasri S, Kiruthika AR, Kumar PS, Yaashikaa PR (2020) Optimization and modeling of reactive yellow adsorption by surface modified Delonixregia seed: study of nonlinear isotherm and kinetic parameters. Surf Interfaces 20:100520
Kosaiyakanon C, Kungsanant S (2020) Adsorption of reactive dyes from wastewater using cationic surfactant-modified coffee husk biochar. Environ Nat Resour J 18(1):21–32
Acknowledgements
Dr. Ali author thanks Prof. Zahra Saleh, Central Laboratories Network and The Centers of Excellence, NRC, for providing instrumental facility.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no external funding.
Author information
Authors and Affiliations
Contributions
The authors are equaly contributed in Conceptualization; Methodology; Formal analysis; Software; Writing—original draft; review & editing.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kandil, H., Ali, H. Simultaneous Removal of Cationic Crystal Violet and Anionic Reactive Yellow Dyes using eco-friendly Chitosan Functionalized by Talc and Cloisite 30B. J Polym Environ 31, 1456–1477 (2023). https://doi.org/10.1007/s10924-022-02682-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02682-0