Abstract
In most wastewater systems, the existence of more than one dye is a reality. Herein, mesoporous iron oxide/polypyrrole/carbon black (Fe3O4/PPy/C) nanocomposite with high saturation magnetization (45.2 emu/g) has been prepared by encapsulating very fine Fe3O4 nanoparticles (4–7 nm) in PPy/C for the first time. The superior adsorption performance of the developed Fe3O4/PPy/C nanocomposite for the removal of basic dye (methylene blue, MB) and acidic dye (Congo red, CR) has been studied in single and binary systems. XRD, FESEM, EDX, HRTEM, FT-IR, VSM, BET, and zeta potential analyses were used for adsorbent characterization. XRD, EDX, and FT-IR findings confirmed the successful inclusion of Fe3O4 into the polymer matrix. The XDR and HRTEM results revealed a significant reduction in the size of the grown Fe3O4 with the addition of PPy/C, which acts as a particle growth inhibitor, and thus the nanocomposite has a greater surface area reached 341.01 m2/g relative to 210.66 m2/g and 277.59 m2/g for pure Fe3O4 and PPy/C, respectively. The Langmuir isotherm matched the experimental data quite well, with R2 and adsorption capacity of 0.994 and 500 mg/g for CR and 0.995 and 90.9 mg/g for MB, respectively. The effectiveness of Fe3O4/PPy/C nanocomposite to remove both CR and MB from binary solution reached 96.9% for CR and 95.9% for MB. In kinetic investigations, the sorption of both dyes obeyed the pseudo-second order model, where R2 equals 0.999 and 0.998 for CR and MB, respectively. Desorption tests revealed the easy separation and good efficiency of the nanocomposite after four cycles. Finally, the sorption mechanism was discussed in detail based on FTIR analysis. Therefore, the developed nanocomposite is a bi-functional, recyclable, and efficient adsorbent for environmental applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It was reported that higher than 650 different contaminants were found in wastewater, including phenols, dyes, pesticides, heavy metals, and pharmaceuticals [1]. Specifically, dyes are organic chemicals that are commonly used to colour paper, plastic, ink, cosmetic, paint, textile, and food [2]. These companies pollute the environment by releasing coloured waste into the environment. Out of 7 × 105 tonnes of commercially used dyes annually generated, around 2.8 × 105 tonnes are dumped into water streams each year [1, 3,4,5]. Dyes in the outflow inhibit photosynthetic activity and, at extremely low concentrations (less than 1 ppm), pose a major harm to the aquatic environment [5,6,7]. Some pigments induce mutations, skin, lung, and cancer when inhaled [1, 3,4,5, 8]. Dyes have thermal, optical, and physicochemical stability due to their complex aromatic structures, making degradability difficult [9]. As a result, dye removal from water sources is critical, and effluent from diverse businesses must be treated before being discharged into the aquatic environment.
There are different kinds of synthetic dyes in waste water. Dyes are classified according to ionic nature, into cationic, anionic, and non-ionic dyes, based on the charge that the dye acquired in its aqueous medium [10]. Congo red (CR) is one of anionic acidic dyes that has high solubility in aqueous environments and thermal stability. In addition, it exhibits resistance against light and biodegradation [5]. The textile, paper, and leather industries are the most common users of this dye. CR is extremely toxic, causing irritation to the skin and eyes, breathing problems, as well as stomach discomfort and blood clotting. It transforms into the carcinogen benzidine during metabolism. This dye was chosen for the for the present investigation because to its danger, strong persistence in the aquatic environment, and the necessity of elimination [1, 3, 5, 11]. Methylene blue (MB) is a cationic soluble dye that widely known as basic blue 9. Like other organic dyes, MB has a variety of negative effects on humans; inhalation can cause difficulty breathing, while injection in the mouth causes burning sensations, vomiting, nausea, diarrhoea, and gasestrics, as well as painful micturation. Because of its wide speared usage as a dye in the textile industry, biological stain, and a range of other uses, in addition to the presence of large amounts of MB in industrial waste [5, 12], MB was chosen as a pollutant in this study.
For this purpose, researchers use a variety of approaches to remove dyes, including photocatalytic degradation [13], catalytic reduction [14], electrochemical oxidation [15], membrane separation [16], and adsorption [17]. All of these approaches necessitate a huge operational investment, as well as a high running cost. Adsorption process was already demonstrated to be a simple, non-toxic, scalable, less byproduct, environmentally friendly, cost-effective, easy reusability of adsorbent, and high-efficiency technology that may be applied efficiently in large-scale industrial dye treatment [1, 8, 9, 18,19,20,21,22,23,24].
Carbon materials, such as activated carbon (AC), carbon nanotubes, graphene and its carbonaceous composites, have attracted researchers' interest as prospective sorbents owing to their large active surface area, high reactivity, porous structure as well as high mechanical and thermal strength and p-p electrostatic interactions [1, 3, 4, 25, 26]. In recent years, the use of carbon nanotubes and graphene oxide showed superior adsorption properties for dye removal when compared to activated carbon [27]. Mishra et al. [28] prepared multiwalled carbon nanotubes by chemical vapour deposition for the adsorption of CR dye. In another study, adsorption of CR was studied by using graphene oxide [29]. Despite the characteristics of such nanomaterials, they have high manufacturing costs and poor separation performance, restricting their scalability [27]. Owing to these disadvantages, researchers are actively looking for low-cost nano-carbonaceous materials with similar features, such as black carbon (CB). CB is mainly composed of elemental carbon (90%), with the rest being oxygen and hydrogen. It has various functional groups, including carbonyl (C=O), hydroxyl (OH), and carboxyl (COOH) [30]. Gaminian and Montazer [31] demonstrated the influence of CB as a filler on the properties of the obtained carbon nanofibers (CFs) derived from cellulose. They demonstrated that the synthesized CB/CFs possess enhanced carbonization yield, thermal stability, electrical conductivity, surface area, and dye adsorption capacity in comparison with CFs obtained from neat cellulose. However, the difficulty in regeneration has not been solved yet. Thus, it is necessary to develop new materials exhibiting strong adsorption and regeneration ability, which is a critical step in the adsorption process.
Magnetic nanoparticles (MNPs) include magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3). Magnetite is the most common magnetic core employed in adsorption investigations, however, it soon oxidizes to maghemite, which is the more stable magnetic oxide in aqueous solution [20]. They possess unique properties, including low toxicity, small particle size, high surface area, biocompatibility, chemical stability, high saturation magnetization, and low synthesis cost [32,33,34]. The magnetic properties of iron oxide based adsorbents enable them to be separated from water after adsorption using gradient magnetic separation technique. As a result, magnetic based sorbents have gained high interest in adsorption because of its remarkable characteristics, including high reusability, easy separation, as well as preserving high efficiency after 3–5 cycles of regeneration [11, 20]. Raw MNPs can be quickly oxidized by the environment and it could leach into low pH conditions, becoming poisonous. In addition, because of their large surface area and energy, they are subject to agglomeration in solutions, which reduces their sorption capacity and limits their use [20]. To compensate for these limitations, much focus has been given to surface modification of MNPs with organic polymer [35], clay [36, 37], surfactant [38], silica [12], activated carbon [39, 40], and etc. For the synthesis of magnetic-based nanocomposites, co-precipitation was recommended as being the most simple, fast, effective, and low-cost technique [37].
Polyaniline (PANI), polypyrrole (PPy), polyethylenimine, and polyacrylonitrile, are nitrogen-containing polymers and possess positive charged sites in which anionic dyes (like CR) can be adsorbed [41]. Among these polymers, PPy is the most frequently applied in various technologies owing to its multifunctional properties, including porous structure, excellent electrical conductivity, great thermal and environmental stability, low toxicity, ease of synthesis, non-localized π-electron conjugate system, and remarkable redox characteristics [18, 42]. As a consequence, it can interact with the target molecule via a variety of intermolecular interactions, including hydrogen bonding, acid–base interactions, and pollutant-polymer exchange [43]. PPy as well as its modified forms have shown great effectiveness in eliminating several common contaminants, as reported in numerous publications. For instance, Xin and co-workers [44] prepared PPy nanofibers with high specific surface area of 86 m2/g and outstanding adsorption capacity (qmax) for methyl orange (MO) dye reached 169.55 mg/g. However, according to Langmuir model qmax of PPy for MB was only 49.7 mg/g as reported by Li et al. [45]. Bhaumik et al. [46] prepared PPy/PANI nanofibres for adsorption of CR (qmax = 222.22 mg/g). Shahnaz et al. [47] modified nanocellulose with PPy for elimination of Cr (VI) and CR dye in binary mixture. Kamal et al. [48] developed a ternary nanocomposite composed of chitosan, graphene oxide, and PPy to eliminate heavy metals (Cd2+, Cu2+) and CR dye. Wang et al. [49] prepared hyper-cross-linked polymer/PPy for efficient phenol adsorption. Nevertheless, separating adsorbent material from aqueous medium is a major source of consternation, restricting how effective adsorbents may be. As a result, the incorporation of magnetic NPs enables magnetic decantation to easily retrieve the nanocomposite from an aqueous solution.
A variety of magnetic polypyrrole based composites have been used for environmental applications. Magnetic NPs can be coated or integrated into a polymer matrix to give colloidal stability and enough protection across a wide range of pH values [50]. Recently, Marghaki et al. [18] studied the isotherms, kinetics, and thermodynamics for the adsorption of Cr (VI) using microbial cellulose/Fe3O4/PPy. Falahian et al. [51] used Fe3O4/PPy composite for Hg2+ elimination. The removal of methyl orange and crystal violet dyes was studied by using polypyrrole modified chitosan-based magsorbent [52]. Recently, Han et al. [53] presented that PPy sensitization enhanced the photocatalytic activity of Fe3O4/SiO2/TiO2 toward degradation of rhodamine B dye. Yang et al. [54] prepared Fe3O4/TiO2/PPy photocatalyst for degradation of methyl orange under ultraviolet and solar radiation. Nayebi and Babamoradi [55] studied the photocatalytic degradation of MB dye using Fe3O4/ZnO/PPy composite. Apart from the environmental applications, iron oxide/polypyrrole based composites are also used in various fields. For example, Li et al. [56] used Fe3O4/PPy/PANI nanocomposite as a microwave absorbing material. Goswami et al. [57] prepared Fe3O4/PPy, Fe3O4/PPy-MB, and Fe3O4/PPy-MO as electrode materials for supercapacitors. Wuang et al. [58] prepared functionalized PPy-Fe3O4 for biomedical applications.
Because both the physicochemical characteristics of the as-prepared adsorbent and the physicochemical properties of the target adsorbate influence adsorption performance, preparing an adsorbent with outstanding properties is a need for success. Competitive adsorption of several types of organic pollutants, such as anionic and cationic dyes, is the normal situation in practical applications. Numerous materials have exhibited good adsorption characteristics in single dye system, however, few only were examined for efficient sorption of different kinds of dyes in binary systems. As a result, we developed new adsorbent with improved adsorption capacity, stability, and recyclability by integrating the advantages of magnetic Fe3O4, along with organic polymer (PPy), and carbon based material for the effective uptake of basic dye (MB) and acidic dye (CR) from single and binary system. Until now, no research has been conducted on the utilization of Fe3O4/PPy/C nanocomposite as an adsorbent for environmental applications, as will be presented in this work. XRD, FTIR, FESEM, EDX, HRTEM, VSM, zeta potential, and BET were used to characterize the samples. The influence of different parameters, including pH, adsorbent dosage, contact time, and initial dye concentration were discussed. The adsorption kinetics and isotherms have been investigated. The reusability potential of Fe3O4/PPy/C nanocomposite is also established. The high adsorptive capability of the nanocomposite is governed by its large surface area, mesoporous structure, high surface roughness, and different function groups. Finally, the sorption mechanism of MB and CR onto the surface of magnetic Fe3O4/PPy/C nanocomposite was discussed in detail based on the FTIR results before and after adsorption of CR and MB dye.
Materials and Methods
Materials
Polypyrrole doped- 20 wt. % composite with carbon black (PPy/C) was purchased from Sigma-Aldrich. Ferrous sulphate and ferric chloride were obtained from Loba Chemie. Congo red (CR) and methylene blue (MB) were supplied by S D Fine-Chem limited and their characteristics are summarized in Table 1.
Synthesis of Fe3O4 and Fe3O4/Polypyrrole/Carbon Black (Fe3O4/PPy/C) Nanocomposite
Iron oxide (Fe3O4) was prepared by co-precipitation method under nitrogen gas. Prepare 2:1 M solution of Fe3+ and Fe2+ ions and then an ammonia solution was added to it to raise pH up to 11 at a temperature of 70 °C and continue stirring for four hours. The resulting black precipitate was centrifuged, washed several times with deionized water, and then dried in a vacuum oven at 50 °C.
The synthesis of Fe3O4/PPy/C nanocomposite was carried out by dispersing 0.4 g of powdered polypyrrole/carbon black (PPy/C) in Fe3+/Fe2+ solution and stirring it for 1 h. Then, follow the same steps as in the case of pure iron oxide synthesis. Scheme 1 revealed the synthesis steps of pure Fe3O4 nanoparticles and Fe3O4/PPy/C nanocomposite.
Characterization
Fourier transform infrared (FTIR) spectra were measured to identify the function groups in the prepared samples by using Bruker instrument, Germany. At 77 K, the nitrogen adsorption/desorption isotherm was measured using Quantachrome apparatus, USA. The specific surface area, average pore diameter, and pore volume were evaluated using Brunauer-Emmett and Teller (BET) method. The surface microstructure and the chemical composition of Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite were identified by using field emission scanning electron microscopy (FESEM, Quanta 250 FEG FEI, United States) and the energy dispersive X-ray spectroscopy (EDX), respectively. X-ray diffraction (XRD) examination employing PAN analytical X-ray diffractometers was used to determine phase purity, structural type, and crystallite size of the materials. The particle size and shape of the samples were determined using high resolution transmission electron microscopy (HR-TEM) utilizing the Jeol JEM2100 series, Japan. At room temperature, the saturation magnetization as well as the coercivity were measured using a vibration sample magnetometer (VSM, MicroMag 3900 series). The applied magnetic field ranged from − 20 to 20 KOe. Zeta potential values (ζ) of samples was measured using a nano Zetasizer analyzer (Malvern, UK) to determine the charge on their surfaces.
Adsorption Experiments
In the batch mode, CR and MB adsorption tests were carried out individually or in mixed solution using a shaker at 200 rpm as follows; 20 mg of Fe3O4/PPy/C nanocomposite was added to 40 mL of dye solution and, at various intervals of time, part of the supernatant was taken and centrifuged. The residual concentration of dye was determined by using spectrophotometer (2401 Shimadzu UV–vis, Japan). The two most important parameters of adsorption are adsorption capacity (qmax) and removal efficiency (RE), which indicate how successful an adsorbent is for removing pollutants. Adsorption capacity is a characteristic of an adsorbent toward the adsorbate, whereas removal efficiency is influenced by the concentration and dose of the adsorbate [20]. Using Eqs. 1 and 2, the dye removal efficiency and the amount of dye adsorbed, respectively, were estimated.
where, Co—initial concentration of each dye (mg/L), Ct—the equilibrium concentration of each dye (mg/L), W– the mass of the nanocomposite (g), and V—the volume of dye solution (L). The influence of different parameters, including pH, adsorbent dosage, contact time, and initial dye concentration were discussed. The kinetics of adsorption were investigated. To explore the detail adsorption behavior and study adsorption isotherms, multiple starting concentrations of CR solution ranged from 40 to 140 mg/L and MB solution (20–60 mg/L) were utilized.
Reusability
The use of magnetic adsorbents is characterized by the simplicity be recovered after use just by applying external magnetic field to nanocomposite-dye suspension solution. The durability studies were conducted by immersing the resultant dye-adsorbed nanocomposite overnight in 0.1 mol/L solution of NaOH and HCl to release CR and MB, respectively, and then washing with distilled water for usage in the subsequent adsorption process. Four rounds of regeneration tests were carried out.
Results and Discussion
Material Characterization
XRD Analysis
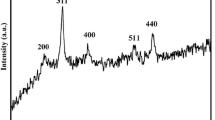
Figure 1 displays the XRD patterns of PPy/C, Fe3O4, and Fe3O4/PPy/C nanocomposite. Pure PPy/C in Fig. 1a has an amorphous broad peak at 25° of 2θ, similar to other common polymers. This peak is attributed to amorphous polypyrrole [51, 59]. The weak peak in the range of 40°–45° is related to amorphous carbon of carbon black [60]. The Fe3O4 characteristic peaks at 18.32o (111), 30.3° (220), 35.6° (311), 43.28° (400), 53.6° (422), 57.18° (511), and 62.8° (440) are demonstrated in the XRD pattern of pure Fe3O4 (Fig. 1b) and Fe3O4/PPy/C nanocomposite (Fig. 1c) (JCPDS Card No 19-629) [61, 62]. This result indicates that the presence of PPy/C particles not affect the crystal structure of the grown Fe3O4 nanoparticles. Furthermore, the Fe3O4/PPy/C nanocomposite has a hump at about 2θ = 25° when compared with pure Fe3O4 as shown in Fig. 1B. This peak is related to polypyrrole based on the literature [59, 63, 64]. Numerous studies demonstrated that the presence of powdered Fe3O4 during the polymerization of pyrrole also had no effect on the crystal structure of the synthesized Fe3O4 particles [59]. Because of the polymer incorporation, the intensity of the Fe3O4 peaks is quite less in the Fe3O4/PPy/C nanocomposite compared to the pristine Fe3O4 sample. Using Scherrer's equation, the main diffraction peak in the XRD pattern was chosen to estimate the crystallite size of the grown Fe3O4 particles. It was found that the crystallite size is 15 nm for pure Fe3O4 sample, whereas it decreased to 11 nm for Fe3O4/PPy/C nanocomposite. This result will be confirmed by HRTEM micrographs.
FESEM and EDX Analyses
The surface and elemental composition of Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite were analyzed by using FESEM and EDX as shown in Fig. 2. The particles of the prepared Fe3O4, Fig. 2a, have smooth and uniform morphology with grain size of 50–80 nm. Figure 2b depicts a FESEM picture of PPy/C, revealing spherical particles with a rough surface. This sample is characterized by large grains varying in size from 70 to 110 nm. The FESEM image of Fe3O4/PPy/C nancomposite at the same magnification is seen in Fig. 2c. The aggregation of the powdered nanocomposite is owing to the magnetic character of the Fe3O4 NPs [35]. The nanocomposite is composed of spherical particles like Fe3O4 rather than larger particles with more pores, different cavities, and a very rough structure. These features are very important for dye adsorption. The EDX spectrum of pure Fe3O4, Fig. 2d, displays the existence of Fe and O atoms only, indicating the high purity of the prepared sample. PPy/C consists of C, N, and O as presented in Fig. 2e. In case of Fe3O4/PPy/C nancomposite, Fig. 2f, the presence of Fe confirming the successful formation of Fe3O4 into the polymer matrix. This analysis agreed with that of XRD results.
The 3D-FESEM images of Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite are shown in Fig. 3. The calculated average surface roughness (Ra) of pure Fe3O4 and PPy/C was 57.9 nm and 136.6 nm, respectively. The low Ra of Fe3O4 is related to its uniform and smooth surface, as shown in Fig. 2a. On the other hand, the surface of Fe3O4/PPy/C nanocomposite is rougher and the value of average surface roughness reached 145.0 nm. The high surface roughness value is corroborated by the large surface area of the composite, as will be illustrated later.
HRTEM Analysis
For more clarity, the morphology of Fe3O4/PPy/C nanocomposite in comparison with pure Fe3O4 was examined by HRTEM. The synthesized Fe3O4 nanoparticles have regular spherical shape with diameter ranged from 9 to 14 nm, Fig. 4a, b, as estimated by using software ImageJ. Pure PPy/C displays spongy node-like structures that are highly interconnected (not shown) [65]. The Fe3O4/PPy/C nanocomposite, Fig. 4c, d, is composed of black spots grouped in some regions in the PPy/C gray area, indicating the growth of Fe3O4 particles that are impeded and strong interacted with the polymer chains. Nevertheless, the presence of PPy/C during the synthesis of Fe3O4 may act as a surfactant or particle growth inhibitor, which causes a significant decrease in the size of the grown Fe3O4 particles to 4–7 nm as revealed by comparing Fig. 4b, d. This phenonium will have an impact on the surface area of the obtained Fe3O4/PPy/C nanocomposite, as will be illustrated in the surface area section. The very common strategy conducted by numerous researches [55, 58] to synthesize magnetic-polypyrrole based composites is that polymerization of pyrrole in the presence of the powder of inorganic nanomaterial in the polymer forming solution, which resulted in an increase in the diameter of the nanoparticles as well as a decrease in the surface area of the obtained composite. Whereas, our modified method, which is based on encapsulation of the powdered polymer in the Fe3O4 synthesis solution, led to a clear decrease in nanoparticle size in addition to an increase in the nanocomposite surface area. Figure 4e, f show the SAED pattern of pure Fe3O4 and Fe3O4/PPy/C nanocomposite, respectively. The creation of clear and brilliant circular rings in both samples, confirming that the presence of polymer does not affect the crystalline structure of the formed Fe3O4 nanoparticles, in agreement with XRD data.
FT-IR Spectroscopy
Figure 5 displays the FT-IR spectra of pure Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite. The spectrum in Fig. 5a showed a sharp absorption peak at ~ 587 cm−1 and small peak at 442 cm−1, which are attributed to the characteristic stretching vibration mode of Fe–O in the prepared Fe3O4 [59, 66]. For PPy/C, Fig. 5b, the large absorption at about 3400 cm−1 corresponding to the N–H and O–H bond stretching. The peaks at 2920 and 2850 cm−1 are attributed to the stretching vibration of the C–H [5, 42, 55]. The bending mode of adsorbed water molecules appeared at 1630 cm−1 [67]. Stretching vibration of C = C appeared at 1542 cm−1 [35]. The 1458 cm−1 band might be attributed to symmetric stretching of pyrrole ring as well as conjugated C–N stretching [35, 68]. The peaks at 1376 and 1165 cm−1 are attributed to the in-plane deformation and bending of C–H of the pyrrole ring, respectively, [69, 70]. At 1041 cm−1, the N–H in-plane deformation appeared [68]. The spectrum of Fe3O4/PPy/C nanocomposite demonstrated the Fe3O4 and PPy/C characteristic peaks. The pyrrole ring peak at 1542 cm−1 is enhanced. The Fe–O stretching peak shifted to 592 cm−1. The peak at ~ 628 cm−1 could be correlated to Fe–O stretching in the Fe3O4 [3, 33]. These results confirmed that Fe3O4 has been successfully introduced into polymer matrix.
VSM Measurement
For wastewater treatment, Fe3O4 nanoparticles serve as effective magnetic carriers for absorbents or photocatalysts, enabling recyclability, reusability, nontoxicity, and low-cost materials [71]. The magnetic behavior of PPy/C, Fe3O4, and Fe3O4/PPy/C nanocomposite at room temperature was investigated using a vibrating sample magnetometer (VSM) as shown in Fig. 6. The values of the saturation magnetization (Ms), coercivity (Hc), and remanent magnetization (Mr) for all samples are summarized in Table 2. The magnetization curve of pristine PPy/C, Fig. 6a, revealed its diamagnetic behavior [72] with very small saturation magnetization (Ms) of 0.176 emu/g. This result confirming the nonmagnetic character of polypyrrole. However, both Fe3O4 and Fe3O4/PPy/C possess a ferromagnetic behavior as presented by the hysteresis loops in the inset of Fig. 6b, c, respectively. The prepared Fe3O4 has very high Ms value of about 73.36 emu/g and the coercivity value (Hc) is about 46.6 Oe. This is equivalent to the magnetization reported using a different synthesis approach (like solvothermal method) to make Fe3O4 nanoparticles have Ms of 82 emu/g [59]. However, the applied co-precipitation method is easier, faster, and more scalable than solvothermal method. Loading the non-magnetic PPy/C on the surface of magnetic nanoparties declines the magnetization to 45.19 emu/g while increasing the coercivity to 53.65 Oe owing to surface heterogeneity and external morphological alteration [73]. Various studies revealed that the sensitization of magnetic nanoparticles with PPy decreased their magnetic features [53, 59, 73]. Despite the fact that the prepared Fe3O4/PPy/C nanocomposite has a higher magnetic value than the majority of previous publications on magnetic Fe3O4/PPy composites [51, 58, 59, 74], an outside magnetic field may separate it from water fast. The relatively low Hc value (< 100) proved that Fe3O4 and Fe3O4/PPy/C possess soft magnetic characters [75].
Surface Area Analysis
Adsorption process is strongly influenced by the adsorbent's surface chemistry. The particle size, specific surface area, pore volume, and pore size of an adsorbent all have a role for efficient adsorption of contaminants [20]. Figure 7A shows the nitrogen adsorption isotherms of Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite. According to Fig. 7a, the specific surface area of the synthesized Fe3O4 was around 210.66 m2/g. When compared to the reported value for Fe3O4 (33.3 m2/g), which was prepared using a solvothermal method, this result is obviously superior [59]. Also, Anjana et al. (Anjana et al., 2018) prepared Fe3O4 by a co-precipitation method; however, the BET surface area was only 48.29 m2/g. The raw PPy/C has 277.59 m2/g specific surface area. This value is eight times higher than the surface area of polypyrrole alone (34.7 m2/g) [58]. Following the addition of PPy/C in the Fe3O4 synthesis solution, the surface area of Fe3O4/PPy/C nanocomposite dramatically increased up to 341.01 m2/g. This might be attributed to the Fe3O4's smaller nanoparticle size, as well as the presence of PPy/C inside the obtained composite, acting as a surfactant or particle growth inhibitor. This value is much higher than the reported surface area of Fe3O4/PPy composites (19.8–10.3 m2/g), which were prepared by oxidative polymerization of polypyrrole in the presence of Fe3O4 nanoparticles [58]. The isotherm of Fe3O4/PPy/C nanocomposite exhibited type IV with H3 hysteresis loop, indicating the formation of highly porous materials [76]. In addition, the pore size distribution curve using the DFT model is displayed in Fig. 7B. PPy/C has small pores of size 5.44 nm, whereas pure Fe3O4 contains large pores with a size of 11.98 nm. The pore volume is 0.5502 cm3/g for PPy/C and 0.9055 cm3/g for Fe3O4. Relative to pure Fe3O4, the incorporation of polymer in the iron oxide synthesis solution to get Fe3O4/PPy/C nanocomposite lowered the pore size to 6.75 nm and pore volume to 0.8623 cm3/g. This behavior is owing to the fact that PPy/C was attached to the surface of Fe3O4, so many pores were partly or entirely occupied. The summary results regarding surface area, pore size, and total pore volume are seen in Table 2. Because of the mesoporous structure and big surface area, Fe3O4/PPy/C would be a good candidate for adsorption of pollutants from water.
Zeta Potential
Zeta potential value (ζ) of all samples, including pure Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite, was determined to assess the surface charge. ζ values of Fe3O4, PPy/C, and Fe3O4/PPy/C nanocomposite were, respectively, + 24.7, − 25.7, and − 20.6. This revealed that the polypyrrole unit interacted with the Fe3O4 nanoparticles via chemical interactions, resulting in a reduction in the zeta potential of the formed Fe3O4/PPy/C nanocomposite. This is in agreement with the findings of previous studies [77, 78].
Adsorption Studies
Adsorption Performance of Fe3O4/PPy/C Nanocomposite Toward Anionic Dye (CR), Cationic Dye (MB), and CR–MB Mixed Solution
To evaluate the adsorption activity of Fe3O4/PPy/C nanocomposite toward anionic and basic dyes, Congo red (CR) and methylene blue (MB), respectively, were chosen. The adsorption experiments were carried out using CR, MB, and their binary mixture. Figure 8 shows the UV–Vis absorption spectra of CR, MB, and CR–MB mixture solution in the presence of Fe3O4/PPy/C nanocomposite. The characteristic peak of CR dye, with initial concentration of 120 mg/L, Fig. 8a, diminished with the passage of time. The CR removal percentage is 94.9% after 180 min. The experimental adsorption capacity is 227.7 mg/g. Obviously, the adsorption for MB (40 mg/L) is faster relative to CR adsorption, as shown in Fig. 8b. In this case, the removal efficiency reached 98.2% within only 120 min. The experimental adsorption capacity for MB is 78.6 mg/g. The simultaneous sorption of anionic dye (CR) and basic dye (MB) using the developed Fe3O4/PPy/C nanocomposite was confirmed by testing the nanocomposite in CR–MB binary mixture as shown in Fig. 8c. The nanocomposite can achieve complete removal of both dyes. At 240 min, the Fe3O4/PPy/C nanocomposite revealed 96.9 ± 2% of decolorization for CR and 95.9 ± 1% elimination for MB.
Influence of Solution pH
The surface charge and functional groups of the adsorbent material could be influenced by the pH of dye solution, resulting in a direct effect on the overall adsorption efficiency. CR and MB removal by Fe3O4/PPy/C nanocomposite in a single dye system were carried out in the pH range of 4 to 10 in order to find the ideal pH. Adsorbent dose was 0.5 g/L and initial concentration of CR and MB was 120 and 40 mg/L, respectively. To alter the pH of dye solutions, NaOH and HCl were used. Figure 9a depicts the percentage of CR and MB removed by Fe3O4/PPy/C nanocomposite at various pH values. As pH rises from 4 to 7, CR removal percentage slightly decreased from 97.5% to 94.9%, whereas the adsorption efficiency significantly decreased to 89.4% at pH 10. According to the previous literature, the sulfonate moiety in CR dye dissociates into negative sulfonic groups (− SO3−) at acidic conditions. As a result electrostatic interaction between positively charged adsorbent surface and dye molecules was induced, consequently enhances the adsorption process. Nevertheless, at high pH, extra OH– ions compete with negatively charged dye molecule for adsorption sites causing a decrease in dye removal percentage. As previously mentioned, pH 7 was the most effective for CR sorption onto graphene oxide [79], montmorillonite [80], and amine-functionalized mesoporous hollow shells [81]. Thus, for the rest of our studies on CR adsorption, we chose to start with a solution with a pH of 7, which is the natural pH for dye solution in addition it is less expensive to study at neutral pH than at acidic or basic pH. For MB removal, Fig. 9a, demonstrated a clear improvement in removal efficiency as pH increases. Changes in surface electrical characteristics may be responsible for the phenomenon. At low pH values, the composite would protonated with H+, resulting in a decrease in negative charge. The declined electronegative composite would inhibit the adsorption of additional electropositive MB molecules. According to these findings, the MB adsorption significantly improved from pH 4 to 7 and remained stable from pH 7 to 10. Therefore, a pH of 8 was selected for the further MB adsorption experiments. This result is in agreement with the previous study on the removal of MB on PEO/Bentonite/PANI [82].
Influence of Adsorbent Dose
To investigate the effect of adsorbent dose, a series of adsorption tests were performed using adsorbent doses ranging from 0.1 to 0.7 g/L while maintaining a constant initial CR and MB dye concentration of 120 and 40 mg/L and a pH of 7.0. As the graph shows, Fig. 9b, increasing the quantity or dose of Fe3O4/PPy/C nanocomposite increased the CR and MB dye uptake. At 0.5 g/L of nanocomposite dosage, an optimum removal percentage value of 95% and 98% was obtained for CR and MB, respectively. After that, it nearly reaches equilibrium, resulting in a much smaller increase in dye removal efficiencies. This could be due to the fact that more active surface sites are available for dye adsorption at higher adsorbent dosages. Therefore, other adsorption experiments were conducted with adsorbent dose of 0.5 g/L. These findings agree with the recently reported [83, 84].
Influence of the Initial Dye Concentration
The predominant trend reported in most of the papers is that increasing the concentration of pollutant declines the removal efficiency. The adsorption efficiency of CR was calculated as a function of increasing dye concentration from 40 to 160 mg/L, as presented in Fig. 9c. The CR elimination percentage stepwise decreased from 99.1% to 83.7% with raising the initial dye concentration. For adsorption of MB the dye concentration changed from 20 to 60 mg/L as shown in Fig. 9c. The MB dye adsorption efficiency declined from 99.6 to 75.6% as the initial concentration of dye increased. These findings are consistent with other studies [4, 85, 86]. According to the researchers, the drop in removal % with rising dye concentration was due to a decrease in the amount of active sites at high initial dye concentration in a given dose of adsorbent material [87, 88]. Consequently, 120 mg/L and 40 mg/L were used in all experiments as the optimal initial concentration of CR and MB, respectively.
Influence of the Interaction Time
In the adsorptive removal of contaminants from aqueous solutions, contact time is crucial. It offers an estimate of how long it takes to reach equilibrium and also defines the kinetics of contaminant removal by nano-adsorbents. Whenever somebody defines the adsorbent's economic value and practical relevance in water treatment, contact time is essential. Figure 9d shows the adsorption percentage of CR and MB with initial concentration of 120 and 40 mg/L, respectively, as a function of contact time (0–240 min) in the presence of Fe3O4/PPy/C nanocomposite. The elimination % improved gradually with increasing contact time in case of CR dye. The same trend was observed for the MB dye. At an early stage, the catalyst surface available to interact with the dye molecules, and hence the dye removal percentage rose dramatically with the passage of time until reaching saturation/equilibrium. After that, the dye removal does not vary much with increasing the contact duration [17, 68]. This behavior is consistent with that being previously observed for the removal of various pollutants such as phenol [49], Pb(II) and Cd(II) [4], Cr (IV) and CR dye [39, 89] as well as MB dye [4, 90].
Equilibrium Adsorption Isotherm Studies
To understand the interaction between the adsorbed molecules, including CR and MB dye, and the sorbent surface (Fe3O4/PPy/C nanocomposite), isotherm investigations were carried out using Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D-R) isotherms as shown in Fig. 10. The parameters of various isotherms were summarized in Table 3.
For monolayer sorption processes, the Langmuir equation is used, and the resultant Eq. 3 [91]. The Langmuir model postulates that all sorption sites are identical, so sorption occurs at discrete/homogeneous sites within the sorbent and the highest adsorption is comparable to a saturated monolayer of adsorbate molecules on the adsorbent's surface.
Ce and qe is the equilibrium dye concentration in mg/L and its corresponding adsorption capacity, respectively. qm is the evaluated Langmuir adsorbing capacity in mg/g and Kl represents the Langmuir adsorption constant in L/mg. In the Langmuir adsorption isotherm plot, Fig. 10a, Ce/qe is drawn versus Ce to can determine the values for qm and Kl. As revealed in Table 3, the Langmuir isotherm has the highest correlation coefficient value, 0.994 for CR and 0.995 for MB, when compared to other isotherm models. This proposes that the adsorption of CR and MB by Fe3O4/PPy/C nanocomposite was mostly monolayer coverage. The highest adsorption capacity (qm) for CR was 500 mg/g and MB was 90.9 mg/g. The Langmuir model revealed the highest regression coefficient for the adsorption of CR onto magnetic poly(glycidyl methacrylate)-grafted-PPy microspheres [92]. Also, this model was the best to describe the adsorption of MB dye onto pure PPy [45] and PPy/sodium alginate [4].
The sorption type or nature is determined by estimating the separation factor (RL) value using Eq. 4.
It is preferable adsorption when the value of RL is between 0 and 1, undesirable at for RL > 1, reversible for RL = 1, and irreversible for RL = 0 [93, 94]. At initial concentration of 120 mg/L and 40 mg/L of CR and MB, respectively, the RL value was 0.028 and 0.004, indicating favorable adsorption of both dyes onto the nanocomposite surface.
Freundlich isotherm designates to multilayer sorption process. The equation of Freundlich model in its linear form is given below, Eq. 5 [95]. The Freundlich model describes the adsorbent's surface heterogeneity.
Freundlich constant is KF ((mg/g)(L/mg)1/n) and n (L/g) is adsorption intensity, which value provides us an idea about the affinity of the process. Their values were determined by plotting lnqe versus lnCe as shown Fig. 10b. When 1/n is less than one, the adsorption is favourable, but when 1/n is greater than one, it is unfavourable; when 1/n is equal to one, the sorption is homogeneous [96, 97]. From Table 3, 1/n is less than 1 for both dyes indicating favourable adsorption.
Temkin's equation has the following linear form [98], Eq. 6. This model assumes that the heat of adsorption of all molecules reduces linearly with increasing adsorption on the adsorbent's surface in addition the adsorption happens with a homogeneous distribution of binding energies until the maximum binding energy is attained [99].
R is the universal gas constant, bt related to the heat of adsorption, and KT denotes equilibrium binding constant (L/mg) that corresponds to the maximum binding energy [100]. As shown in Fig. 10c, we can estimate the values of bT and KT by plotting qe against lnCe. Table 3 demonstrates that the Temkin model is not the best suited model, since its R2 value was 0.976 and 0.886 for CR and MB, respectively. The calculated value of binding energy (bT) was 30.5 J/mol for CR and 292.5 J/mol for MB. These values correspond to the physical characteristics of the sorption process.
The linear form of the Dubinin–Radushkevich (D-R) isotherm is presented as follows [97], Eq. 7. It assumed that adsorption occurs on both homogeneous and heterogeneous surfaces.
qm is the estimated adsorption capacity by D-R model and β is the D-R isotherm constant. ε is the Polanyi adsorption, which is calculated using Eq. 8. This model helps to evaluate mean sorption energy (E) using Eq. 9.
The type of adsorption is determined according to the mean sorption energy value, where the E is less than 8 kJ/mol suggesting that the sorption of CR and MB is a physical process [4].
Adsorption Kinetics
The reaction-limiting stage of the sorption process is determined by the adsorption kinetics. Kinetics of dye adsorption were performed using CR solution with initial concentrations ranged from 80 to 140 mg/L and MB solution separately with initial concentrations of 20–60 mg/L. The experimental results were fitted to the pseudo first order (PFO) (Eq. 10), pseudo second order (PSO) (Eq. 11), Intra- particle diffusion (IPD) (Eq. 12), and Elovich model (Eq. 13) and their equations are given as follows.
where K1, K2, and Ki, is the PFO, PSO, and IPD rate constant, respectively. qe—the equilibrium adsorption capacity and qt—adsorption capacity at time t. C—the boundary diffusion influence [101]. α and β are constants of the Elovich model. Figure 11 shows the kinetic models, including PFO, PSO, IPD, and Elovich, for sorption of CR and MB onto Fe3O4/PPy/C nanocomposite.
Table 4 displays the values of kinetic parameters as well as rate constants. The high correlation coefficient (R2) of the PSO model (0.999 for CR and 0.998 for MB) reflects that the kinetics of CR and MB adsorption on nanocomposite were better expressed by PSO model. Furthermore, the calculated adsorption cabacity from this kinetic model is very close to the experimental values. Generally, the pseudo-second-order (PSO) model is the most commonly used model for describing the sorption kinetics of pollutants [17]. The results suggest that chemisorption phenomena are involved in the whole adsorption process for CR and MB dye indicating electron exchange between adsorbate and adsorbent molecules [102]. PSO had a calculated adsorption capacity of 333.3 mg/g for CR and 83.3 mg/g for MB. The calculated k2 of MB adsorption was higher than that of CR adsorption, indicating the faster MB adsorption onto the surface of Fe3O4/PPy/C than CR, which agrees with the obtained results. Yu et al. [92] recently reported that the PSO model best describes CR adsorption onto magnetic poly(glycidyl methacrylate)-grafted-PPy microspheres. Also, studying the kinetics of the adsorption of CR dye onto γ-Fe2O3/PPY indicated that it is a chemisorption process [35]. For the PFO model, the calculated adsorption capacities not comparable with the experimental one in addition the relatively low R2 value that indicating the adsorption processes not obey the pseudo-first order. From the data in Table 4 for IPD model, it was found that C ≠ 0 for adsorption of both CR and MB onto Fe3O4/PPy/C nanocomposite. This revealed that intraparticle diffusion isn’t the sole rate controlling step and the dye adsorption processes not fitted the IPD model. The Elovich model assumes that the active sites of a sorbent are heterogeneous. The constants of Elovich model α and β, respectively, represent the initial adsorption rate and the desorption constant. The obtained R2 values indicated that the Elovich model didn’t accurately describe the adsorption processes on the nanocomposite.
Reusability Study
The reusability of Fe3O4/PPy/C nanocomposite as an adsorbent for CR and MB was studied for individual dye solution to assess its economic efficiency. The adsorbent dosage was 0.5 g/L and the used concentration of CR and MB was 120 and 40 mg/L, respectively. After the first adsorption cycle, external magnetic field was used to collect the dye adsorbed nanocomposite. 0.1 M solution of NaOH and HCl was used to liberate CR and MB dye, respectively. Then wash with distilled water for usage in the subsequent adsorption procedure. Four rounds of regeneration tests were carried out and the corresponding removal efficiency was estimated for each dye as presented in Fig. 9e. According to the results, after the fourth cycles, the nanocomposite still exhibited more than 85% removal efficiency. This indicates that the developed magnetic nanocomposite has good reusability and stability.
Proposed Adsorption Mechanism
FTIR analysis was conducted to understand the adsorption mechanism since it reveals the functional groups in the adsorbent material and their interaction locations. Figure 12 reveals the FT-IR spectra of Fe3O4/PPy/C nanocomposite before and after adsorption of CR (CR-Fe3O4/PPy/C) and MB (MB-Fe3O4/PPy/C). After dye adsorption, there are small shift and a broadening in the peaks of N–H and O–H at about 3400 cm−1 and 1630 cm−1 indicating association of these function groups in dye adsorption process, which could be by hydrogen bonding with the adsorbed dye [63, 103]. The characteristic peaks of polypyrrole in the range of 1560 and 1350 cm−1 are obviously broadened, confirming incorporation of the pyrrole ring with the adsorbed dyes, which may be due to π-π interaction between the aromatic ring PPy and that of adsorbed dyes. Also, Bhaumik et al. [46] reported that after CR adsorption, the peaks of PPy/PANI are slightly moved towards a lower wavenumber and their intensity is lowered. In comparison to fresh nanocomposite, the FT-IR spectrum of CR or MB after adsorption over Fe3O4/PPy/C nanocomposite showed minor shifts in band locations and a decrease in intensity of the Fe–O stretching peaks 592 and 628 cm−1 [3, 33]. This demonstrates involvement of Fe3O4 in the dye adsorption process, which could be through surface complexation. These results confirmed our proposed adsorption mechanism for CR and MB on the surface of Fe3O4/PPy/C nanocomposite, Scheme 2.
Physical adsorption or chemical adsorption or a combination of both, generally, is the possible adsorption strategies based on the type of interaction between the adsorbent and adsorbate [104]. Adsorption mechanism of CR and MB onto the surface of Fe3O4/PPy/C nanocomposite may be attributed to a variety of interactions, such as π-π interaction, hydrogen bonding, electrostatic interaction, and surface complexation [1, 3, 45, 57, 103], as presented in Scheme 2. (i) π-π interaction may occur between the aromatic ring of CR or MB dye with that of PPy in the nanocomposite [17, 57, 104]. (ii) The N (nitrogen) atom of the dye (C-N group), which is highly electronegative and has lone pair of electrons, can act as a hydrogen bonding acceptor and form hydrogen bonds with the H-atom of the hydroxyl groups of the PPy/C backbone. Owing to the difference in the chemical structure of each dye, CR dye can form more hydrogen bonds than MB dye, leading to higher adsorption affinity toward CR dye. (iii) The sulphonate group in CR dye is negatively charged and thus can interact with metallic cationic moieties (ζ of Fe3O4 = + 24.7), leading to electrostatic attraction between nanocomposite and CR dye [35, 92]. Also, there is electrostatic attraction between the positively charged MB dye and the net negative charge on the surface of the nanocomposite (ζ of Fe3O4/PPy/C = − 20.6). (IV) Finally, surface complexation between nanocomposite and CR dye. It was reported that the task of Fe3O4 for the sorption of CR in Fe3O4-based composites is that Fe3O4 helps in the creation of a complex between the molecules of CR dye and the composite [103]. Also, the existence of Fe(II) assists chemisorption sites, consequently facilitating bonding of anionic dye (CR) [1, 103]. In addition, Afkhami and Moosavi [105] attributed the CR adsorption onto γ-Fe2O3 to the coordination interaction between metal ions and amine groups of CR molecules. Accordingly, different forces are involved in the adsorption mechanism of CR and MB onto the Fe3O4/PPy/C nanocomposite as displayed in Scheme 2.
The proposed mechanism is supported by the dye adsorption findings. (1) The experimental dye adsorption results indicated that the synthesized nanocomposite exhibited a higher adsorption affinity toward CR than MB dye. This agrees with the proposed adsorption mechanism where the higher combination interaction boosts CR adsorption more than in the case of MB dye. (2) According to adsorption kinetic studies, the PSO model exhibited the highest correlation coefficient (R2) (0.999 for CR and 0.998 for MB). This indicates that chemisorption phenomena are involved in the whole adsorption process for both CR and MB dye, in agreement with the proposed adsorption mechanism. (3) The value of the PSO rate constant (k2) of MB adsorption was higher than that of CR adsorption, indicating the faster MB adsorption onto the surface of Fe3O4/PPy/C than CR in accordance with adsorption results, Fig. 8. (4) The interpretation of isotherm results indicated that the adsorption of CR and MB by Fe3O4/PPy/C nanocomposite follows Langmuir isotherm owing to the highest correlation coefficient value, 0.994 for CR and 0.995 for MB. The the sorption type is determined by estimating the separation factor (RL) value, which was 0.028 and 0.004 for CR and MB, respectively, indicating favorable adsorption of both dyes onto the nanocomposite surface. (5) The mean sorption energy (ED-R), which determined from the Dubinin–Radushkevich (D-R) isotherm, for the sorption of CR and MB is less than 8 kJ/mol suggesting that the adsorption takes place physically [4]. According to the zeta potential results, the surface of nanocomposite is negatively charged thus it can be predicted that the nanocomposite exhibits higher adsorption properties toward positively charged MB dye than negatively charged CR dye. On the other hand, experimentaly the nancomposite showed larger adsorption capacity for anionic CR dye revealing that the electrostatic attractive forces not playing a prominent role in the adsorption process. There are many adsorption processes follwed the PSO kinetic model eventhough ED-R is less than 8 kJ/mol. For example, the adsorption of MB dye on sodium alginate/polypyrrole composite followed the Langmuir isotherm model and PSO kinetic model, however the ED-R value was 0.24 kJ/mol [4]. The authors implied that the adsorption of MB onto the adsorbent might take place via a variety of mechanisms, such as Van der Waals, hydrogen bonding, electrostatic interactions, and π-π interactions. Recently, Sarojini et al. [1] studied the adsorption on CR dye on polypyrrole/iron oxide/seaweed (PPy/Fe3O4/SW) nanocomposite. They found that the PSO model fits the kinetics of CR adsorption, the CR adsorption followed the Langmuir isotherm, and the calculated ED-R was very low, E = 0.853 kJ/mol. In addition, Ekka et al. [21] found that the Cr(VI) adsorption was well fitted with the Langmuir isotherm model and ED-R = 2.64 kJ/mol. However, PSO was the best to descripe the kinetics of adsorption, R2 = 0.999. Similar findings were reported by other authors [22, 106,107,108,109]. To sum up, the adsorption characteristics of the prepared Fe3O4/PPy/C nanocomposite more likely be controlled by a number of different interactions cumulatively apart from electrostatic attractive forces there are hydrogen bonding, π–π interaction, and surface complexation may also contribute to the adsorption features of the nanocomposite.
Comparative Study
Table 5 compares the characteristics of the developed Fe3O4/PPy/C nanocomposite with various adsorbents in terms of saturation magnetization, specific surface area, and the adsorption capacity toward CR and/or MB dye. These adsorbents are classified into three groups as follows. The adsorbents in group A can uptake CR dye only. The adsorbents in group B can remove both CR and MB dye. For group C, these materials can remove MB only. Relative to these adsorbents, it is clear that the prepared Fe3O4/PPy/C nanocomposite has higher adsorption capacity. There are some adsorbents have comparable adsorption capacities of the nanocomposite, but the Fe3O4/PPy/C exhibited more enhanced magnetic properties compared to these adsorbents. In conclusion, the advantages of the Fe3O4/PPy/C nanocomposite in comparison with other adsorbents are large surface area, mesoporous structure, high adsorption capacity, sorption ability toward cationic and anionic dyes, as well as simple separation owing to high magnetization. Obviously, the synthesized nanocomposite has potential application for treating wastewater.
Conclusions
In this paper, the newly Fe3O4/PPy/C nanocomposite was prepared by encapsulating very fine Fe3O4 nanoparticles (4–7 nm) in PPy/C polymer matrix. XRD and HRTEM proved that the inclusion of PPy/C in the iron oxide synthesis solution causes a clear decrease in the size of the grown Fe3O4 nanoparticles. It is suggested that the polymer works as a surfactant or particle growth inhibitor. Magnetic analyses demonstrate that saturation magnetization (Ms) and coercivity (Hc) values are 45.201 emu/g and 54.03 Oe for Fe3O4/PPy/C nanocomposite and 73.365 emu/g and 47.4 Oe for uncoated Fe3O4, respectively. In comparison with pure Fe3O4 and PPy/C, the developed Fe3O4/PPy/C nanocomposite has higher surface roughness (Ra = 145.0 nm) and specific surface area (341.01 m2/g). The adsorption ability of the nanocomposite was evaluated using CR, MB, and CR–MB binary mixture solution. In single dye solution, the effect of different adsorption parameters such as pH, contact time, adsorbent dosage, and initial dye concentration as well as the sorption kinetics and isotherms were investigated. It was found that the Langmuir model and the pseudo-second order model well described the sorption of organic dyes onto the nanocomposite surface. The as-prepared nanocomposite had a high adsorption capacity, reaching 500 mg/g for CR and 90.9 mg/g for MB. The reusability potential of Fe3O4/PPy/C nanocomposite is also established. The proposed adsorption mechanism for the uptake of CR and MB is based on the FTIR results after dye adsorption. The adsorption of CR on Fe3O4/PPy/C nanocomposite is due to π–π stacking, hydrogen bonding, electrostatic attraction, and surface complexation, whereas there are only three types of interaction that are involved in the MB adsorption, including π–π stacking, hydrogen bonding, and electrostatic attraction. This may explain the higher removal efficiency for anionic CR dye than MB. Considering the characteristics of Fe3O4/PPy/C, including large surface area, high rough surface, mesoporous structure, rich functional groups, and high magnetization, it may be used to efficiently remove various pollutants.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Sarojini G, Babu SV, Rajasimman M (2022) Adsorptive potential of iron oxide based nanocomposite for the sequestration of Congo red from aqueous solution. Chemosphere 287:132371
Mittal J (2020) Permissible synthetic food dyes in India. Resonance 25:567–577
Priyan VV, Kumar N, Narayanasamy S (2022) Toxicological assessment and adsorptive removal of lead (Pb) and Congo red (CR) from water by synthesized iron oxide/activated carbon (Fe3O4/AC) nanocomposite. Chemosphere 294:133758–133758
Heybet EN, Ugraskan V, Isik B, Yazici O (2021) Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int J Biol Macromol 193:88–99
Anuma S, Mishra P, Bhat BR (2021) Polypyrrole functionalized Cobalt oxide Graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J Hazard Mater 416:125929
Deb A, Debnath A, Bhowmik K, Paul SR, Saha B (2021) Application of polyaniline impregnated mixed phase Fe2O3, MnFe2O4 and ZrO2 nanocomposite for rapid abatement of binary dyes from aqua matrix: response surface optimization. Int J Environ Anal Chem 1−19.
Mittal J, Ahmad PR, Mittal A (2021) Kahwa tea (Camellia sinensis) carbon—a novel and green low-cost adsorbent for the sequestration of titan yellow dye from its aqueous solutions. Desalin Water Treat 227:404–411
Paul SR, Singh NH, Debnath A (2022) Quick and enhanced separation of Eosin Yellow dye from aqueous solution by FeCl3 interaction: thermodynamic study and treatment cost analysis. Int J Environ Anal Chem 1−21.
Arora C, Kumar P, Soni S, Mittal J, Mittal A, Singh B (2020) Efficient removal of malachite green dye from aqueous solution using Curcuma caesia based activated carbon. Desalin Water Treat 195:341–352
Mittal J (2021) Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: a review. J Environ Manag 295:113017
Pi Y, Duan C, Zhou Y, Sun S, Yin Z, Zhang H, Liu C, Zhao Y (2022) The effective removal of Congo Red using a bio-nanocluster: Fe3O4 nanoclusters modified bacteria. J Hazard Mater 424:127577
Lei Y, Zhang X, Meng X, Wang Z (2022) The preparation of core-shell Fe3O4@ SiO2 magnetic nanoparticles with different surface carboxyl densities and their application in the removal of methylene blue. Inorg Chem Commun 139:109381
Ali H, Mansor ES (2020) Co-sensitization of mesoporous ZnS with CdS and polyaniline for efficient photocatalytic degradation of anionic and cationic dyes. Colloid Interface Sci Commun 39:100330
Benhadria N, Hachemaoui M, Zaoui F, Mokhtar A, Boukreris S, Attar T, Belarbi L, Boukoussa B (2022) Catalytic reduction of methylene blue dye by copper oxide nanoparticles. J Cluster Sci 33(1):249–260
Mohan N, Balasubramanian N, Basha CA (2007) Electrochemical oxidation of textile wastewater and its reuse. J Hazard Mater 147(1–2):644–651
Mansor ES, Ali H, Abdel-Karim (2020) A efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloid Interface Sci Commun 39:100314
Prajapati AK, Mondal MK (2022) Green synthesis of Fe3O4-onion peel biochar nanocomposites for adsorption of Cr (VI), methylene blue and congo red dye from aqueous solutions. J Mol Liq 349:118161
Marghaki NS, Jonoush ZA, Rezaee A (2022) Chromium (VI) removal using microbial cellulose/nano-Fe3O4@ polypyrrole: isotherm, kinetic and thermodynamic studies. Mater Chem Phys 278:125696
Jayalakshmi R, Jeyanthi J, Sidhaarth KA (2022) Versatile application of cobalt ferrite nanoparticles for the removal of heavy metals and dyes from aqueous solution. Environ Nanotechnol Monit Manag 17:100659
Emenike EC, Adeniyi AG, Omuku PE, Okwu KC, Iwuozor KO (2022) Recent advances in nano-adsorbents for the sequestration of copper from water. J Water Process Eng 47:102715
Ekka B, Dhar G, Sahu S, Mishra M, Dash P, Patel RK (2021) Removal of Cr (VI) by silica-titania core-shell nanocomposites: in vivo toxicity assessment of the adsorbent by Drosophila melanogaster. Ceram Int 47(13):19079–19089
Saha B, Debnath A, Saha B (2022) Fabrication of PANI@ Fe–Mn–Zr hybrid material and assessments in sono-assisted adsorption of methyl red dye: uptake performance and response surface optimization. J Indian Chem Soc 99(9):100635
Kumar V, Saharan P, Sharma AK, Umar A, Kaushal I, Mittal A, Al-Hadeethi Y, Rashad B (2020) Silver doped manganese oxide-carbon nanotube nanocomposite for enhanced dye-sequestration: Isotherm studies and RSM modelling approach. Ceram Int 46(8):10309–10319
Mittal J, Ahmad R, Mariyam A, Gupta VK, Mittal A (2021) Expeditious and enhanced sequestration of heavy metal ions from aqueous environment by papaya peel carbon: a green and low-cost adsorbent. Desalin Water Treat 210:365–376
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK (2021) Fixed-bed adsorption of the dye Chrysoidine R on ordered mesoporous carbon. Desalin Water Treat 229:395–402
Saharan P, Kumar V, Mittal J, Sharma V, Sharma AK (2021) Efficient ultrasonic assisted adsorption of organic pollutants employing bimetallic-carbon nanocomposites. Sep Sci Technol 56(17):2895–2908
Cai Z, Sun Y, Liu W, Pan F, Sun P, Fu J (2017) An overview of nanomaterials applied for removing dyes from wastewater. Environ Sci Pollut Res 24:15882–15904
Mishra AK, Arockiadoss T, Ramaprabhu S (2010) Study of removal of azo dye by functionalized multi walled carbon nanotubes. Chem Eng J 162:1026–1034
Sahoo SK, Sahoo JK, Panigrahi GK, Pattanayak DK, Rout AS, Lenka A (2020) Preparation of graphene oxide from Bio-soot wastes: as an efficient adsorbent for highly noxious Congo red dye. FlatChem 24:100198
Zappielo CD, Nanicuacua DM, dos Santos WN, da Silva DL, Dall’Antônia LH, Oliveira FMD, Clausen DN, Tarley CR (2016) Solid phase extraction to on-line preconcentrate trace cadmium using chemically modified nano-carbon black with 3-mercaptopropyltrimethoxysilane. J Braz Chem Soc 27:1715–1726
Gaminian H, Montazer M (2018) Carbon black enhanced conductivity, carbon yield and dye adsorption of sustainable cellulose derived carbon nanofibers. Cellulose 25(9):5227–5240
Lei C, Wen F, Chen J, Chen W, Huang Y, Wang B (2021) Mussel-inspired synthesis of magnetic carboxymethyl chitosan aerogel for removal cationic and anionic dyes from aqueous solution. Polymer 213:123316
Anjana PM, Bindhu MR, Umadevi M, Rakhi RB (2018) Antimicrobial, electrochemical and photo catalytic activities of Zn doped Fe3O4 nanoparticles. J Mater Sci 29(7):6040–6050
Ch´avez-Guajardo AE, Maqueira L, Medina-Llamas JC, Alcaraz-Espinoza J, Araújo TL, Vinhas GM, Rodrigues AR, Alves KG, De Melo CP (2015) Use of magnetic and fluorescent polystyrene/tetraphenylporphyrin/maghemite nanocomposites for the photoinactivation of pathogenic bacteria. React Funct Polym 96:39–43
Ramírez-Ortega AA, Medina-Llamas M, da Silva RJ, García-Elías J, de Lira-Gómez P, Medina-Llamas JC, Chávez-Guajardo AE (2021) Synthesis of a maghemite-polypyrrole nanocomposite for the removal of congo red dye from aqueous solutions. Environ Nanotechnol Monit Manag 16:100597
Saikia PK, Sarmah PP, Borah BJ, Saikia L, Saikia K, Dutta DK (2016) Stabilized Fe3O4 magnetic nanoparticles into nanopores of modified montmorillonite clay: a highly efficient catalyst for the Baeyer-Villiger oxidation under solvent free conditions. Green Chem 18:2843–2850
Abdullah NH, Shameli K, Abdullah EC, Abdullah LC (2020) Low cost and efficient synthesis of magnetic iron oxide/activated sericite nanocomposites for rapid removal of methylene blue and crystal violet dyes. Mater Charact 163:110275
Zhao W, Zhao Y, Zhang H, Hao C, Zhao P (2022) Efficient removal of cationic and anionic dyes by surfactant modified Fe3O4 nanoparticles. Colloids Surf A 633:127680
Ren L, Lin H, Meng F, Zhang F (2019) One-step solvothermal synthesis of Fe3O4@ Carbon composites and their application in removing of Cr (VI) and Congo red. Ceramics 43(7):9646–9652
Juang RS, Yei YC, Liao CS, Lin KS, Lu HC, Wang SF, Sun AC (2018) Synthesis of magnetic Fe3O4/activated carbon nanocomposites with high surface area as recoverable adsorbents. J Taiwan Inst Chem Eng 90:51–60
Bhat SA, Zafar F, Mondal AH, Mirza AU, Haq QMR, Nishat N (2020) Efficient removal of Congo red dye from aqueous solution by adsorbent films of polyvinyl alcohol/melamine-formaldehyde composite and bactericidal effects. J Clean Prod 255:120062
Agrawal R, Shah J, Gupta G, Srivastava R, Sharma C, Kotnala R (2020) Significantly high electromagnetic shielding effectiveness in polypyrrole synthesized by eco-friendly and cost-effective technique. J Appl Polym Sci 137(48):49566
Li X, Miao J, Yin Z, Xu X, Shi H (2019) Polypyrrole-modified nylon 6 nanofibers as adsorbent for the extraction of two b-lactam antibiotics in water followed by determination with capillary electrophoresis. Molecules 24:2198
Xin Q, Fu J, Chen Z, Liu S, Yan Y, Zhang J, Xu Q (2015) Polypyrrole nanofibers as a high-efficient adsorbent for the removal of methyl orange from aqueous solution. J Environ Chem Eng 3:1637–1647
Li J, Feng J, Yan W (2013) Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for Methylene Blue. Appl Surf Sci 279:400–408
Bhaumik M, McCrindle R, Maity, (2013) A Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibres. Chem Eng J 228:506–515
Shahnaz T, Padmanaban VC, Narayanasamy S (2020) Surface modification of nanocellulose using polypyrrole for the adsorptive removal of Congo red dye and chromium in binary mixture. Int J Biol Macromol 151:322–332
Kamal S, Khan F, Kausar H, Khan MS, Ahmad A, Ahmad SI, Asim M, Alshitari W, Nami SAA (2020) Synthesis, characterization, morphology, and adsorption studies of ternary nanocomposite comprising graphene oxide, chitosan, and polypyrrole. Polym Compos 41(9):3758–3767
Wang X, Ou H, Huang J (2019) One-pot synthesis of hyper-cross-linked polymers chemically modified with pyrrole, furan, and thiophene for phenol adsorption from aqueous solution. J Colloid Interface Sci 538:499–506
Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ (2015) Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev 44:6733–6748
Falahian Z, Torki F, Faghihian H (2018) Synthesis and application of polypyrrole/Fe3O4 nanosize magnetic adsorbent for efficient separation of Hg2+ from aqueous solution. Global Chall 2(1):1700078
Mashkoor F, Nasar A (2020) Facile synthesis of polypyrrole decorated chitosan-based magsorbent: characterizations, performance, and applications in removing cationic and anionic dyes from aqueous medium. Int J Biol Macromol 161:88–100
Han KH, Kim YH, Mun MH, Yu JH, Han RH (2022) Synthesis of polypyrrole-modified Fe3O4/SiO2/TiO2 nanocomposite microspheres and their photocatalytic activity. Mater Res Express 9(2):025007
Yang X, Zhang L, Chen Z, Jing H, Chen Y, Li Q (2016) Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole. Sci Eng Compos Mater 23(3):269–275
Nayebi P, Babamoradi M (2021) Synthesis of ZnO nanorods/Fe3O4/polypyrrole nanocomposites for photocatalytic activity under the visible light irradiation. Optik 244:167497
Li B, Weng X, Wu G, Zhang Y, Lv X, Gu G (2017) Synthesis of Fe3O4/polypyrrole/polyaniline nanocomposites by in-situ method and their electromagnetic absorbing properties. J Saudi Chem Soc 21(4):466–472
Goswami B, Das C, Mahanta D (2021) Effect of dye-adsorption on Fe3O4-polypyrrole nanocomposite as electrode material in electrochemical capacitors. J Energy Storage 44:103429
Wuang SC, Neoh KG, Kang ET, Pack DW, Leckband DE (2007) Synthesis and functionalization of polypyrrole-Fe3O4 nanoparticles for applications in biomedicine. J Mater Chem 17(31):3354–3362
Park DE, Chae HS, Choi HJ, Maity A (2015) Magnetite–polypyrrole core–shell structured microspheres and their dual stimuli-response under electric and magnetic fields. Mater Chem C 3(13):3150–3158
Shi X, Zhang J, Huang T (2018) The key roles of trace iron for nitrogen, sulfur dual-doped carbon nanospheres as high efficient oxygen reduction catalyst. J Mater Sci 53(2):1404–1413
Sun X, Zheng C, Zhang F, Yang Y, Wu G, Yu A, Guan N (2009) Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe (III) precursor by a sucrose bifunctional hydrothermal method. J Phys Chem C 113(36):16002–16008
Xuan S, Wang YXJ, Leung KCF, Shu K (2008) Synthesis of Fe3O4@ polyaniline core/shell microspheres with well-defined blackberry-like morphology. J Phys Chem C 112(48):18804–18809
Senguttuvan S, Janaki V, Senthilkumar P, Kamala-Kannan S (2022) Polypyrrole/zeolite composite—a nanoadsorbent for reactive dyes removal from synthetic solution. Chemosphere 287:132164
Sahu S, Kar P, Bishoyi N, Mallik L, Patel RK (2019) Synthesis of polypyrrole-modified layered double hydroxides for efficient removal of Cr (VI). J Chem Eng Data 64(10):4357–4368
Faisal M, Rashed MA, Ahmed J, Alsaiari M, Jalalah M, Alsareii SA, Harraz FA (2022) Au nanoparticles decorated polypyrrole-carbon black/g-C3N4 nanocomposite as ultrafast and efficient visible light photocatalyst. Chemosphere 287:131984
Zhu M, Diao G (2011) Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J Phys Chem C 115(39):18923–18934
Aliabadi RS, Mahmoodi NO (2018) Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. J Clean Prod 179:235–245
Wang Y, Chen R, Dai Z, Yu Q, Miao Y, Xu R (2022) Facile preparation of a polypyrrole modified Chinese yam peel-based adsorbent: characterization, performance, and application in removal of Congo red dye. RSC Adv 12(15):9424–9434
Ansar MT, Ali A, Mustafa GM, Afzal F, Ishaq S, Kanwal F, Naseem S, Atiq S (2021) Polypyrrole-based nanocomposites architecture as multifunctional material for futuristic energy storage applications. J Alloys Compd 855:157341
Dhananjayan N, Jeyaraj W, Karuppasamy G (2019) Interactive studies on synthetic nanopolymer decorated with edible biopolymer and its selective electrochemical determination of L-tyrosine. Sci Rep 9(1):1–12
Nguyen MD, Tran HV, Xu S, Lee TR (2021) Fe3O4 nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl Sci 11(23):11301
Aravindan N, Kanagaraj M, Sangaranarayanan MV (2016) Tuning the magnetic and structural properties of electrodeposited nickel–polypyrrole (Ni-PPy) composites through moderate stirring. Mater Chem Phys 174:6–10
Li Y, Yi R, Yan A, Deng L, Zhou K, Liu X (2009) Facile synthesis and properties of ZnFe2O4 and ZnFe2O4/polypyrrole core-shell nanoparticles. Solid State Sci 11(8):1319–1324
Chen W, Li X, Xue G, Wang Z, Zou W (2003) Magnetic and conducting particles: preparation of polypyrrole layer on Fe3O4 nanospheres. Appl Surf Sci 218(1–4):216–222
Appaturi JN, Pulingam T, Muniandy S, Dinshaw IJ, Fen LB, Johan MR (2019) Supported cobalt nanoparticles on graphene oxide/mesoporous silica for oxidation of phenol and electrochemical detection of H2O2 and Salmonella spp. Mater Chem Phys 232:493–505
Lu X, Lin Y, Dong H, Dai W, Chen X, Qu X, Zhang X (2017) One-step hydrothermal fabrication of three-dimensional MoS2 nanoflower using polypyrrole as template for efficient hydrogen evolution reaction. Sci Rep 7(1):1–8
Sahu S, Bishoyi N, Patel RK (2021) Cerium phosphate polypyrrole flower like nanocomposite: a recyclable adsorbent for removal of Cr (VI) by adsorption combined with in-situ chemical reduction. J Ind Eng Chem 99:55–67
Sahu S, Bishoyi N, Sahu MK, Patel RK (2021) Investigating the selectivity and interference behavior for detoxification of Cr (VI) using lanthanum phosphate polyaniline nanocomposite via adsorption-reduction mechanism. Chemosphere 278:130507
Debnath S, Maity A, Pillay K (2014) Impact of process parameters on removal of Congo red by graphene oxide from aqueous solution. J Environ Chem Eng 2(1):260–272
Yermiyahu Z, Lapides I, Yariv S (2005) Thermo-XRD-analysis of montmorillonite treated with protonated Congo-red. Curve fitting. Appl Clay Sci 30(1):33–41
Velmurugan P, Shim J, Oh BT (2016) Removal of anionic dye using amine-functionalized mesoporous hollow shells prepared from corn cob silica. Res Chem Intermed 42(6):5937–5950
Ali H, Mansor ES, Taha GM (2022) Microfiltration and adsorptive membranes for simultaneous removal of methyl orange and methylene blue using hybrid composites. Polym Bull 79(9):7891–7908
Das P, Nisa S, Debnath A, Saha B (2022) Enhanced adsorptive removal of toxic anionic dye by novel magnetic polymeric nanocomposite: optimization of process parameters. J Dispersion Sci Technol 43(6):880–895
Daraei H, Mittal A (2017) Investigation of adsorption performance of activated carbon prepared from waste tire for the removal of methylene blue dye from wastewater. Desalin Water Treat 90:294–298
Das P, Debnath P, Debnath A (2021) Enhanced sono-assisted adsorptive uptake of malachite green dye onto magnesium ferrite nanoparticles: kinetic, isotherm and cost analysis. Environ Nanotechnol Monit Manag 16:100506
Mittal J, Mariyam A, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Batch and bulk adsorptive removal of anionic dye using metal/halide-free ordered mesoporous carbon as adsorbent. J Cleaner Prod 321:129060
Ali H, Ismail AM (2022) Structural, morphological, and enhanced dye sorption properties of mesoporous TiO2/ZnS nanocomposite synthesized by SILAR method. J Inorg Organomet Polym Mater 32(1):326–343
Ismail AM, Menazea AA, Ali H (2021) Selective adsorption of cationic azo dyes onto zeolite nanorod-based membranes prepared via laser ablation. J Mater Sci 32(14):19352–19367
Alok M, Jyoti M, Arti M, Gupta VK (2009) Adsorptive removal of hazardous anionic dye" Congo red" from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340(1):16–26
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Efficient batch and Fixed-Bed sequestration of a basic dye using a novel variant of ordered mesoporous carbon as adsorbent. Arabian J Chem 14(6):103186
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361–1368
Yu Y, Su J, Liu J, Li W (2022) Magnetic poly (glycidyl methacrylate) microspheres with grafted polypyrrole chains for the high-capacity adsorption of Congo Red Dye from aqueous solutions. Coatings 12(2):168
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK (2021) Adsorption behaviour of Chrysoidine R dye on a metal/halide-free variant of ordered mesoporous carbon. Desalin Water Treat 223:425–433
Mittal A, Thakur V, Mittal J, Vardhan H (2014) Process development for the removal of hazardous anionic azo dye Congo red from wastewater by using hen feather as potential adsorbent. Desalin Water Treat 52(1–3):227–237
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Jain R, Sharma P, Sikarwar S, Mittal J, Pathak D (2014) Adsorption kinetics and thermodynamics of hazardous dye Tropaeoline 000 unto Aeroxide Alu C (Nano alumina): a non-carbon adsorbent. Desalin Water Treat 52(40–42):7776–7783
Ali H, Ismail AM (2021) Developing montmorillonite/PVDF/PEO microporous membranes for removal of malachite green: adsorption, isotherms, and kinetics. J Polym Res 28:429
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Phys Chem URSS 12:217–256
Ahmad R, Hasan I, Mittal A (2017) Adsorption of Cr (VI) and Cd (II) on chitosan grafted polyaniline-OMMT nanocomposite: isotherms, kinetics and thermodynamics studies. Desalin Water Treat 58:144–153
Patel A, Soni S, Mittal J, Mittal A, Arora C (2021) Sequestration of crystal violet from aqueous solution using ash of black turmeric rhizome. Desalin Water Treat 220:342–352
Soni S, Bajpai PK, Bharti D, Mittal J, Arora C (2020) Removal of crystal violet from aqueous solution using iron based metal organic framework. Desalin Water Treat 205:386–399
Arab C, El Kurdi R, Patra D (2022) Zinc curcumin oxide nanoparticles for enhanced adsorption of Congo red: kinetics and adsorption isotherms study. Mater Today Chem 23:100701
Sirajudheena P, Nikithaa MR, Karthikeyana P, Meenaksh S (2020) Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene oxide–carboxymethyl cellulose recyclable composite: adsorption investigation of parametric studies and their mechanisms. Surf Interfaces 21:10648
Bai L, Li Z, Zhang Y, Wang T, Lu R, Zhou W, Gao H, Zhang S (2015) Synthesis of water-dispersible graphene-modified magnetic polypyrrole nanocomposite and its ability to efficiently adsorb methylene blue from aqueous solution. Chem Eng J 279:757–766
Afkhami A, Moosavi R (2010) Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J Hazard Mater 174:398–403
Elwakeel KZ, Elgarahy AM, Elshoubaky GA, Mohammad SH (2020) Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J Environ Health Sci Eng 18(1):35–50
Munir M, Nazar MF, Zafar MN, Zubair M, Ashfaq M, Hosseini-Bandegharaei A, Khan SUD, Ahmad A (2020) Effective adsorptive removal of methylene blue from water by didodecyldimethylammonium bromide-modified Brown clay. ACS Omega 5(27):16711–16721
Lafi R, Montasser I, Hafiane A (2019) Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorp Sci Technol 37(1–2):160–181
Chen D, Zeng Z, Zeng Y, Zhang F, Wang M (2016) Removal of methylene blue and mechanism on magnetic γ-Fe2O3/SiO2 nanocomposite from aqueous solution. Water Resour Ind 15:1–13
Subhan F, Aslam S, Yan Z (2019) Effective adsorptive performance of Fe3O4@SiO2 core shell spheres for methylene blue: kinetics, isotherm and mechanism. J Porous Mater 26:1465–1474
Koohi P, Rahbar-kelishami A, Shayesteh H (2021) Efficient removal of congo red dye using Fe3O4/NiO nanocomposite: synthesis and characterization. Environ Techno Innovation 23:101559
Zhu HY, Fu YQ, Jiang R, Yao J, Xiao L, Zeng GM (2012) Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: preparation, characterization and application for adsorption of dye from aqueous solution. Bioresour Technol 105:24–30
Li L, Li X, Duan H, Wang X, Luo C (2014) Removal of Congo Red by magnetic mesoporous titanium dioxide-graphene oxide core-shell microspheres for water purification. Dalton Trans 43:8431–8438
Alzahrani FM, Alsaiari NS, Katubi KM, Amari A, Rebah FB, Tahoon MA (2021) Synthesis of polymer-based magnetic nanocomposite for multi-pollutants removal from water. Polymers 13(11):1–16
Gupta VK, Agarwal S, Ahmad R, Mirza A, Mittal J (2020) Sequestration of toxic congo red dye from aqueous solution using ecofriendly guar gum/activated carbon nanocomposite. Int J Biol Macromol 158:1310–1318
ZabihiSahebi A, Koushkbaghi S, Pishnamazi M, Askari A, Khosravi R, Irani M (2019) Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr (VI), As (V), Methylene blue and Congo red from aqueous solutions. Int J Biol Macromol 140:1296–1304
Yang L, Zhang Y, Liu X, Jiang X, Zhang Z, Zhang T, Zhang L (2014) The investigation of synergistic and competitive interaction between dye Congo red and methyl blue on magnetic MnFe2O4. Chem Eng J 246:88–96
Arora C, Soni S, Sahu S, Mittal J, Kumar P, Bajpai PK (2019) Iron based metal organic framework for efficient removal of methylene blue dye from industrial waste. J Mol Liq 284:343–352
Soni S, Bajpai PK, Mittal J, Arora C (2020) Utilisation of cobalt doped Iron based MOF for enhanced removal and recovery of methylene blue dye from waste water. J Mol Liq 314:113642
Qi L, Jiaqi Z, Yimin D, Danyang L, Shengyun W, Ling C (2020) Facile synthesis of 5- aminoisophthalic acid functionalized magnetic nanoparticle for the removal of methylene blue. J Mater Sci 31(1):457–468
Acknowledgements
Prof. Zahra Saleh, Central Laboratories Network and The Centers of Excellence, NRC, is acknowledged for her valuable support and help.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work wasn’t funded by any funding agency.
Author information
Authors and Affiliations
Contributions
HA: conceptualization; methodology; data curation; formal analysis; software; visualization; writing—review and editing. AMI: data curation; formal analysis; software; writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, H., Ismail, A.M. Fabrication of Magnetic Fe3O4/Polypyrrole/Carbon Black Nanocomposite for Effective Uptake of Congo Red and Methylene Blue Dye: Adsorption Investigation and Mechanism. J Polym Environ 31, 976–998 (2023). https://doi.org/10.1007/s10924-022-02663-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02663-3