Abstract
The spread of microbes which cause infectious diseases are of great concern on human health. Therefore, a water-soluble cross-linked polymer based on polyvinyl alcohol was synthesized via an economical, facile, and aqueous-based approach. The resultant cross-linked polymer was characterized by different techniques such as FTIR, 1H NMR, 13C NMR, TGA, and DSC. The IR spectrum has been recorded in the range 400–4000 cm−1. From thermal studies, i.e. TGA, cross-linking polymer PVA-E-Pz showed two step degradation and from DSC, glass transition temperature (Tg) was exhibited at 86.05 °C. The antimicrobial properties of the cross-linked polymer were studied using the well-diffusion technique and optical density method against gram-negative bacteria, Escherichia coli and gram-positive bacteria, Staphylococcus aureus. Polymer coated fabric was also evaluated for antimicrobial activity against both the bacteria, even after 25 wash cycles the coated fabric showed about 90% antibacterial activity. Samples showed good antimicrobial activity against both the micro-organisms, but more activity was exhibited against gram-negative bacteria. The coating durability and surface morphology of the coated fabric were also analyzed. Cytotoxicity studies revealed that PVA-E-Pz was non-toxic against human dermal fibroblast cell lines. This material might be a good fit for advanced wound dressing and textile applications. The proposed strategy provides a low-cost, environmentally friendly method for creating a new cross-linked polymer with antimicrobial activity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections are caused by microbes such as fungus, bacteria and parasites [1]. Pathogenic microorganisms are a major source of infections in a variety of fields, namely hospital surfaces/furniture, drugs, medical devices, dental restoration, health care products, surgery equipment, and hygienic applications (such as food packaging and storage, textiles, large or household items etc.) [2,3,4,5,6,7,8,9]. Controlling and preventing microbial diseases becomes a formidable problem because microbes live in every place and may spread via air, food, and water etc. [10,11,12]. Antimicrobial agents, such as disinfectants, antibiotics and antiseptics, have been produced extensively to combat microbial infections [13,14,15,16,17]. However, the extensive and incautious use of disinfectants and antibiotics has resulted in the rise of new antimicrobial-resistant bacterium strains, posing a significant increase in antimicrobial challenges [18,19,20,21]. Low molecular weight compounds cause environmental contamination and are toxic since they are susceptible to resistance [22, 23]. To overcome these problems of low molecular weight compounds, antimicrobial polymers which enhance antimicrobial efficacy and reduce toxicity can be used [13, 24, 25].
The growth of microbes can be prevented by using antimicrobial polymers which are capable of killing or inhibiting their growth [3]. Antimicrobial polymers are non-volatile, chemically stable and exhibit long-term activity [13, 26]. They can be prepared either by using synthetic polymers or by natural polymers. Antimicrobial polymers can be synthesized either by grafting bioactive agents to the polymers via hydrogen or covalent bonding nor by synthesizing a polymer which possess biologically active functional groups namely NH2, Cl, NO2 etc. [27]. These antimicrobial polymers minimize the environmental problems and enhance the efficiency of some existing low molecular weight agents.
Coating antimicrobial polymers on the surfaces inhibit the growth of microbes, by fighting against them on contact [28, 29]. Biofilm formation can also be prevented by coating the surfaces with antimicrobial polymers [29]. As an alternative for antibiotic, new materials or surface coatings have been developed to prevent the adhesion of bacteria onto the surface [28]. Surface coatings can be done using various techniques such as physical vapour deposition, chemical vapour deposition, dip coating, spin coating and spray coating etc. [30]. Antimicrobial coating on surfaces reduces the bacterial adhesion thereby reducing the spread of microbes which is the major reason for causing numerous effects on human health. Polymers due to their physicochemical properties are used as an substituent of glass, wood and metals in various applications [1].
Poly (vinyl alcohol) (PVA) is most abundant synthetic water-soluble polymer [31]. Due of its biodegradability, biocompatibility, water processability, chemical resistance, and great physical qualities, ready availability and low-price it is a widely used polymer with various industrial uses [32,33,34]. PVA has wide range of applications as food packaging materials, coating agents in paper industry, pharmaceuticals, cosmetics and textiles. However, PVA has several disadvantages such as high degree of water absorption, low tensile strength, and high water solubility. To overcome these drawbacks, and to improve the stability and ideal properties of PVA, it is modified as required for different applications [35]. PVA is either chemically or physically altered, or it is combined with other polymers [33]. It has previously been shown that PVA can be blended with antibacterial agents and nanoparticles [35,36,37]. But, no report on the alteration of a PVA through direct covalent bonding of piperazine has been found to our knowledge. A cross-linker is a substance capable of forming chemical bonds and joining polymer chains to one another. When a cross-linker is used in the synthesis, the straight chain is converted into a cross-linked structure, resulting in a stronger structure. The physical characteristics of the polymer will be affected by the cross-linker, such as molecular weight is increased, the elasticity of the polymer is reduced, and the polymer stability is enhanced [38]. Cross-linked polymers can be derived by using various cross-linking agents like epichlorohydrin, glutaraldehyde, borax etc. [39, 40]. In this work, epichlorohydrin is used as cross-linking agent for modification of PVA with piperazine. Various heterocyclic compounds can be used as antimicrobial agents and piperazine is one such compound which exhibits wide range of pharmacological activities like antioxidant, antibacterial, anticancer, antifungal, antiviral, antitubercular activities etc. [41, 42]. Piperazine is a six-membered heterocyclic compound having two nitrogen atoms at opposite positions in the ring [41]. Due to wide range of pharmacological properties of piperazine and its derivatives, they are used in the manufacture of new drugs, chemical industry [43, 44].
Many studies on the fabrication of antimicrobial polymers have been published in past decades [45,46,47]. Pour et al., blended PVA with quaternary ammonium modified starch using glutaraldehyde as cross-linker and citric acid as plasticizer to develop cross-linked PVA films. These films exhibited good antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Bacillus subtilis [48]. Chen et al., developed transparent antimicrobial films by blending PVA with poly(hexamethylene guanidine) and studied their antimicrobial activity against E. coli and S. aureus [49]. Kruszkowska et al., fabricated bioactive materials by modification of PVA with poly(hexamethylene guanidine) and evaluated their antibacterial activity against E. coli and S. aureus [50]. The current article demonstrates the modification of polyvinyl alcohol with piperazine to produce cross-linking polymer which exhibits efficient antimicrobial activity against S. aureus, and E. coli. Further, this study aims to develop antibacterial cotton fabrics coated by prepared cross-linking polymer.
Experimental
Materials
Polyvinyl alcohol (Sigma Aldrich, Average MW 85,000–1,24,000, 99 + %), piperazine (Sigma Aldrich, 99%) and epichlorohydrin (Merck, ≥ 99%) were used as such. Deionized water was used as solvent for the reaction. Ethanol was purchased from changshu and used as received. Plain weave cotton fabric in the experiment was purchased from local industry.
Synthesis of Polyvinyl Alcohol-Epichlorohydrin-Piperazine (PVA-E-Pz) Cross-Linking Polymer
PVA modified with epichlorohydrin and piperazine were synthesized following the previously reported method [51]. PVA, epichlorohydrin and piperazine were dissolved in deionized water in 1 (PVA monomeric unit):1:1 molar ratio. To this appropriate amount of KOH solution was added followed by stirring at 50 °C temperature for about 2 days under nitrogen atmosphere [40, 51]. Further this solution was poured to ethanol and product was precipitated (PVA-E-Pz). The precipitated product was washed with ethanol to remove the unreacted epichlorohydrin, piperazine and any copolymer of epichlorohydrin and piperazine formed (Scheme 1). The obtained product was dried using vacuum oven at 75 °C. The yield obtained was about 61%.
Preparation of Antimicrobial Cotton Fabrics
Cotton fabrics were cut into discs with 1 cm diameter. Initially, they were washed with distilled water followed by sonication with ethanol and acetone for 15 min. These fabrics were then dried. The treatment polymer was then prepared by using 30 mg of PVA-E-Pz/mL of water. The dried cotton fabrics were then coated with prepared treatment polymer by dip coating method. These polymer coated fabrics were then dried and studied for antimicrobial activity, surface morphology and durability.
Characterizations
NMR Analysis
Bruker spectrometer was used to record 1H NMR and 13C NMR spectra with TMS and deuterated DMSO as an internal reference and solvent respectively.
FTIR Analysis
FTIR spectra of PVA-E-Pz cross-linking polymer were acquired using a Shimadzu-8400S spectrometer.
TGA Analysis
TGA thermogram of PVA-E-Pz cross-linking polymer was studied under inert atmosphere using TA TGA 55 instrument over a temperature range 20–800 °C at a heating rate of 10 °C/min using platinum pans.
DSC Analysis
DSC thermogram of PVA-E-Pz was done under inert atmosphere using NETZSCH DSC 404F1 instrument over a temperature range 20–550 °C at a heating rate of 10 °C/min using aluminium pans.
Antimicrobial Activity
Antimicrobial screening for the cross-linked polymer PVA-E-Pz was determined by well-diffusion method. Antibacterial activity against 12 h old Gram-negative E. coli and Gram-positive S. aureus was determined by measuring the zone of inhibition.
Both S. aureus and E. coli were grown in nutrient broth media, incubated at 28–30 °C at 120 rpm for 12 h and this 12 h old bacterial culture was used for the antibacterial activity of PVA-E-Pz. Further, nutrient agar medium was prepared, autoclaved and was poured into the petriplates and kept inside the laminar air flow to solidify. The bacterial cultures were serially diluted upto 10–4 dilution with autoclaved distilled water. 50 µL of 10–4 dilution was inoculated on the agar media and spread using a L-shape spreader. Further, three holes with a diameter of 6 mm are punched aseptically with a sterile cork borer [52,53,54]. 10 mg, 20 mg and 30 mg of PVA and PVA-E-Pz was dissolved in 1 mL of sterile water and a volume of 100 µL of the sample was introduced into the wells. Then, the plates were kept for incubation at 28–30 °C for 12 h and zone of inhibition was measured. Antibacterial activity of the standard (ciprofloxacin) as control was measured and compared with the test sample.
Antimicrobial Activity by Optical Density Method
Furthermore, the antimicrobial activity of PVA-E-Pz was studied by the optical density method using a Varioskan flash spectrometer at 600 nm wavelength. Initially, different wells of 96 well plate was filled with 50 µL of various amounts of PVA-E-Pz cross-linking polymer (0.5, 0.25, 0.125 and 0.0625 mg). Then to each well 100 µL of nutrient broth and 50 µL of diluted bacterial culture was added (Total volume 200 µL). At 600 nm initial optical density was taken for 0 h followed by final optical density i.e. after the incubation of plates at room temperature for 24 h. For standard, 50 µL of serially diluted ciprofloxacin (2.5 mg/mL) was used and for control, 50 µL of water was used in place of sample. Further, percentage inhibition was calculated using the formula:
where ODi and ODf is the difference between initial and final optical density with sample and control.
Cytotoxicity Assay
The cytotoxicity of the synthesized PVA-E-Pz cross-linked polymer was examined using human primary dermal fibroblast cell line (ATCC, USA). In 200 µL of DMEM containing fetal bovine serum, HDF cells were planted in a 96-well plate at the needed cell density (20,000 cells/well) and allowed to grow for roughly 24 h. Further, polymer samples of various concentrations (1,5, 10, 20, and 30 µg/mL) were added to it and incubated in a 5% CO2 atmosphere for 24 h at 37 °C. The plates were removed from the incubator after the incubation period, the used medium was taken out, and MTT reagent was added to form the final concentration of 0.5 mg/mL of total volume. The MTT reagent was withdrawn from the plates after 3 h of incubation, and 100 µL of solubilization solution (DMSO) was then added. By gently swirling in a gyratory shaker, dissolution was enhanced. The percentage cell viability was calculated in comparison with the assay controls.
SEM Analysis
The polymer coated on fabric is observed using a scanning electron microscopy. SEM images were captured in ZEISS EVO MA18 with varied pressure modes at a 20 kV working voltage.
Antimicrobial Activity of Coated Fabric
The bacterial plates were prepared as described above. The polymer coated fabrics developed by dip coating method were placed on the bacteria inoculated plates and incubated at at 28–30 °C for 24 h. Then the antimicrobial activity was observed.
Washing Cycle Measurements
Washing cycle measurements were done according to the procedure reported elsewhere [55]. Initially, sample of known weight was dissolved and coated on cotton fabric, dried and weighed (M1). Further, the fabric was immersed for sometime in water bath at 50 °C, dried in vacuum oven and weighed. The same procedure was repeated for 5, 10, 15, 20 and 25 cycles. The coated fabric was weighed after each of these washing cycles (M2). The below equation is used to calculate weight loss:
Evaluation of Antimicrobial Activity After Washing
Washing durability was evaluated by monitoring the antibacterial activity of PVA-E-Pz coated cotton fabric after subjecting to several washing cycles. The washing of cotton fabric was done by immersing the coated fabric into water bath maintained at 50 °C. After 25 washing cycle the antibacterial activity of the fabric was evaluated against E. coli and S. aureus.
Results and Discussion
NMR and FTIR Analysis
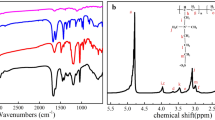
The 1H NMR spectrum of PVA and PVA-E-Pz is shown in Figs. 1 and 2. The chemical shifts in 1H NMR of PVA: δ = 1.31–1.63 ppm corresponds to the proton of CH2 of polymer backbone (1), δ = 3.83–3.86 ppm corresponds to the proton of CH of PVA polymer backbone (2) and δ = 4.27, 4.52, 4.69 ppm are due to the presence of OH group (Fig. 1). The chemical shifts in 1H NMR of PVA-E-Pz: δ = 1.44–1.49 ppm corresponds to proton of CH2 present in the polymer backbone (2), δ = 2.19–2.46 ppm is due to proton of CH2 attached to N of piperazine (6,7), δ = 2.84 ppm is due to proton of CH attached to O (1), δ = 3.72–3.89 ppm is due to proton of CH and CH2 attached to O (3,4,5). The peak around 4.6 ppm is attributed to OH and intensity of this peak is less compared to that of PVA due to the cross-linking (Fig. 2).
The 13C NMR spectrum of PVA and PVA-E-Pz is shown in Figs. 3 and 4. The chemical shifts in 13C NMR of PVA: δ = 45.13–46.66 ppm corresponds to carbon of CH2 present in polymer backbone (2), δ = 64.23–68.45 ppm corresponds to carbon of CH present in polymer backbone (1) (Fig. 3). The chemical shifts in 13C NMR of PVA-E-Pz: δ = 43.94–46.61 ppm corresponds to carbon of CH2 present in polymer backbone (2), δ = 52.81–53.91 ppm corresponds to carbon of CH2 attached to N of piperazine (6,7), δ = 63.31–66.39 ppm, 68.18 ppm corresponds to carbon of CH, CH2 attached to O (1,3,4,5) (Fig. 4).
In FTIR spectrometry, analysis was carried out to approve the presence of characteristic functional groups as well as the interaction between the starting materials to form the synthesized compounds. Figure 5a & b shows the FTIR spectrum of PVA and PVA-E-Pz respectively. The bands appeared at 3289.2 cm−1 and 2910.9 cm−1 is attributed to O–H stretching and –CH stretching of PVA (Fig. 5a). Whereas in PVA-E-Pz the O–H stretching was observed at 3276.2 cm−1 which was slightly lower than O–H stretching of PVA which may be due to some hydrogen bond force in PVA-E-Pz [56]. The bands at 2902.0 cm−1 and 2827.8 cm−1 is attributed to –CH stretching of PVA and piperazine [57], respectively. Meanwhile, the characteristic peaks at 1274.6 cm−1 and 945.5 cm−1 is attributed to –CN stretching and ring skeleton structure of piperazine [58, 59] (Fig. 5b).
Thermal Analysis
The thermogram of the PVA-E-Pz is depicted in Fig. 6. PVA-E-Pz showed two-step degradation at 287.89 °C and 406.15 °C. The first weight loss stage starts at about 30 °C which may be due to the presence of water. The rate of mass loss is found to be 26.99%, 31.67% and 25.24% respectively. The half decomposition temperature of the polymer i.e. the temperature at which half weight loss occurred was 310.63 °C.
According to weight loss data, weight loss occurs as soon as the heat treatment is started. Three weight losses can be observed from 26.02 to 170.26 °C, 287.89–324.49 °C and 406.15–470.91 °C respectively as show in Table 1. The ability of a polymeric material to withstand the action of heat is known as thermal stability [60]. From TGA thermogram, it is found that PVA-E-Pz is thermally stable upto 470.91 °C and then it decomposes.
The DSC thermogram of PVA and PVA-E-Pz are given in Fig. 7. Whenever a polymer is studied as a measure of temperature, the first important thermal response is the glass transition, resulting to a change to the rubbery phase from glass phase [61]. The glass transition temperature (Tg) of PVA and cross-linking polymer PVA-E-Pz is observed to be 76.40 °C and 86.05 °C. During the formation of cross-linking polymer the intermolecular interactions between the chains increases and Tg value also increases [62]. Thus, a slight shift is observed in the position of Tg value of PVA-E-Pz when compared to PVA. The amount of energy required to break the interactions between the polymer and the cross-linker determines the bond strength of the interaction. If the interaction is stronger, more endothermic energy is required to break the interactions [63]. The cross-linking polymer PVA-E-Pz exhibited multiple endothermic peaks T1Endo, T2Endo, T3Endo, T4Endo and T5Endo which were observed at 95.07 °C, 205.60 °C, 304.65 °C, 431.46 °C and 507.11 °C respectively.
Antimicrobial Activity
The synthesized cross-linking polymer exhibited significant zone of inhibition against S. aureus and E. coli.
The zone of inhibition (diameter) of the cross-linking polymer PVA-E-Pz against S. aureus and E. coli is given in table (Table 2). Figure 8 shows the antimicrobial activity of PVA and PVA-E-Pz at different concentrations 10 mg, 20 mg and 30 mg for S. aureus and E. coli. PVA does not exhibit any antimicrobial activity, whereas for PVA-E-Pz, as the concentration increases, the zone of inhibition diameter also increases. PVA-E-Pz shows better antimicrobial activity against E. coli than S. aureus. The higher activity of PVA-E-Pz against E. coli may be due to the presence of thinner cell wall than S. aureus [64, 65]. PVA-E-Pz’s antibacterial properties is due to the presence of piperazine, which affects the cytoplasmic membrane of bacteria, allowing intercellular components to flow out and eventually causing cell lysis [56, 66]. To compare the results, antimicrobial activity of ciprofloxacin (10 mg) was measured and it exhibited a zone of inhibition of 45 mm and 46 mm against S. aureus and E. coli, respectively.
Antimicrobial Activity by Optical Density Method
Percentage inhibition of S. aureus and E. coli was calculated using Eq. (1) and was represented graphically with respect to the standard (Fig. 9). From the calculation, it was found that PVA-E-Pz at different amounts (0.5, 0.25, 0.125 and 0.0625 mg) showed a percentage inhibition of 94.35%, 92.03%, 89.18% and 86.68% against S. aureus and 96.05%, 93.92%, 91.85% and 89.74% against E. coli respectively. The percentage inhibition exhibited against E. coli is higher than that exhibited against S. aureus for all the tested amounts. As the amount of PVA-E-Pz decreased the percentage inhibition was also decreased and it is observed that the minimum inhibitory concentration of PVA-E-Pz < 0.0625 mg. The obtained results are in good agreement with the zone of inhibition exhibited by PVA-E-Pz against S. aureus and E. coli.
Cytotoxicity Assay
Non-cytotoxicity of the modified polymer is important for application point of view. To study the effects of modified polymer on human skin, cytotoxicity assay were carried on human fibroblast cell line. The observations of MTT assay illustrated that PVA-E-Pz showed moderate cytotoxicity on HDF cells with 75% of cell viability at highest concentration i.e. 30 µg/mL. According to the results, after 24-h treatment period, the samples that showed cell viability of more than 75% were determined to be non-toxic to human dermal fibroblasts (Fig. 10).
SEM Analysis of Coated Cotton Fabric
The surface morphology of the treated and untreated cotton fabric at various magnifications is shown in Fig. 11. The cotton fabric which is treated had a quite rough surface when compared to untreated cotton fabric which is smooth. The change in surface of modified cotton fabric was due to the presence of PVA-E-Pz. The layer of material seen on the treated cotton fabric indicates that PVA-E-Pz has been coated on the surface of treated cotton fabric.
Antimicrobial Activity of Coated Fabric
Based on the experimental results of antimicrobial activity of PVA-E-Pz, the antimicrobial efficacy of the coated fabric was also studied. It was found that the coated fabric substrate after exposure to S. aureus and E. coli for 24 h exhibited bacterial inhibition which reveals that PVA-E-Pz cross-linking polymer is an excellent candidate for antibacterial surface preparation (Fig. 12).
Durability of Coated Fabrics
The durability of the treated fabric is an important property that must be examined after the treatment. The results obtained are shown in table given (Table 3). This is done by calculating the weight loss of modified fabric after they've been through several washing cycles using Eq. (2). From the data given in Table 3, it was found that the weight loss percentage increased somewhat as the number of washing cycles increased. In first three washing cycles majority of loss of coating occurred. This is probably because unbound or weakly connected PVA-E-Pz were easily removed in the first few washing cycles, leaving the strongly attached PVA-E-Pz which is difficult to remove in subsequent washing processes. It may be observed that about 80% of the coating remained on the cotton fabric even after 25 washing cycles. Durability of polymer coated fabric may be due to two reasons: Crosslinked polymer PVA-E-Pz is not easily soluble in water and hydrogen bonding between PVA-E-Pz and the cotton fabric. From the results obtained it is revealed that the coated fabric is durable.
Effect of Washing on Fabric’s Antimicrobial Activity
Washing durability is an important parameter for PVA-E-Pz coated cotton fabric. In this work, cotton fabric coated with PVA-E-Pz was put through 25 washing cycle to demonstrate the durability of the antibacterial activity against washing cycle. The modified cotton fabrics are durable for the washing process and the antibacterial rate for E. coli and S. aureus is above 90% even after 25 washing cycles. These results indicated that increase in washing cycles has small effect on the antibacterial activity of PVA-E-Pz coated cotton fabric.
Conclusion
This research focused on the investigation of antimicrobial activity of cross-linking polymer PVA-E-Pz prepared via. one-pot synthesis and eco-friendly method. PVA has wide range of coating applications, thus we modified it using piperazine by easy and efficient method. The structure of synthesized cross-linking polymer PVA-E-Pz was confirmed by FTIR, NMR (1H and 13C). The thermal studies revealed that PVA-E-Pz shows two step degradation and glass transition temperature (Tg) is at 86.05 °C. Further, antimicrobial activity of PVA-E-Pz was studied against Escherichia coli and Staphylococcus aureus by well-diffusion method. Particularly, PVA-E-Pz displayed efficient antimicrobial activity against gram-negative bacteria, E. coli (17–34 mm) compared to gram-positive bacteria, S. aureus (14–18 mm). This may be due to the presence of thin cell wall of E. coli compared to S. aureus. Further from optical density method it was found that minimum inhibitory concentration of PVA-E-Pz < 0.625 mg. Cytotoxicity studies on human fibroblast cell lines revealed the synthesized cross-linked polymer was non-toxic.
The antibacterial activity and surface morphology of the PVA-E-Pz coated fabric was also studied. The polymer coated fabric’s durability to washing cycle was also investigated and the results revealed that even after 25 washing cycles, 80% of coating were retained on fabric. It was also found that about 90% antibacterial activity of coated fabric was retained even after 25 washing cycles. Taken as a whole, these findings suggest that introduction of piperazine into PVA, display good antibacterial activity and thus could be used for textile or wound dressing applications. This study suggests that employing PVA-E-Pz in antimicrobial applications might pave the way for greater research into generating more effective antimicrobial polymers in the future.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Irshad K, Rehman K, Sharif H, Akash MSH (2020) Antimicrobial polymer coating. Polymer coatings. Wiley, Hoboken, pp 347–358
Nagaraja A, Puttaiahgowda YM, Devadiga D (2019) Synthesis and fabrication of high-potent antimicrobial polymeric ultrathin coatings. J Appl Polym Sci 136:47893. https://doi.org/10.1002/app.47893
Jain A, Duvvuri LS, Farah S et al (2014) Antimicrobial polymers. Adv Healthc Mater 3:1969–1985. https://doi.org/10.1002/adhm.201400418
Bshena O, Heunis TDJ, Dicks LMT, Klumperman B (2011) Antimicrobial fibers: therapeutic possibilities and recent advances. Future Med Chem 3:1821–1847. https://doi.org/10.4155/fmc.11.131
Emam HE (2019) Antimicrobial cellulosic textiles based on organic compounds. 3 Biotech 9:29. https://doi.org/10.1007/s13205-018-1562-y
Gottenbos B (2001) Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 48:7–13. https://doi.org/10.1093/jac/48.1.7
Page K, Wilson M, Parkin IP (2009) Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Mater Chem 19:3819. https://doi.org/10.1039/b818698g
Park E-S, Lee H-J, Park HY et al (2001) Antifungal effect of carbendazim supported on poly(ethylene-co-vinyl alcohol) and epoxy resin. J Appl Polym Sci 80:728–736. https://doi.org/10.1002/1097-4628(20010502)80:5%3c728::AID-APP1149%3e3.0.CO;2-7
Patel MB, Patel SA, Ray A, Patel RM (2003) Synthesis, characterization, and antimicrobial activity of acrylic copolymers. J Appl Polym Sci 89:895–900. https://doi.org/10.1002/app.11970
Camacho-Cruz LA, Velazco-Medel MA, Cruz-Gómez A, Bucio E (2021). Antimicrob Polym. https://doi.org/10.1007/978-981-15-7098-8_1
Naebe M, Haque ANMA, Haji A (2021) Plasma-assisted antimicrobial finishing of textiles: a review. Engineering. https://doi.org/10.1016/j.eng.2021.01.011
Kivumbi MT, Standley CJ (2021) Efforts to identify and combat antimicrobial resistance in Uganda: a systematic review. Trop Med Infect Dis 6:86. https://doi.org/10.3390/tropicalmed6020086
Xue Y, Xiao H, Zhang Y (2015) Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int J Mol Sci 16:3626–3655. https://doi.org/10.3390/ijms16023626
Namivandi-Zangeneh R, Wong EHH, Boyer C (2021) Synthetic antimicrobial polymers in combination therapy: tackling antibiotic resistance. ACS Infect Dis 7:215–253. https://doi.org/10.1021/acsinfecdis.0c00635
Ventola CL (2015) The antibiotic resistance crisis: causes and threats: part 1: causes and threats. Pharm Ther 40:277–283
Berendonk TU, Manaia CM, Merlin C et al (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317. https://doi.org/10.1038/nrmicro3439
Hay SI, Rao PC, Dolecek C et al (2018) Measuring and mapping the global burden of antimicrobial resistance. BMC Med 16:78. https://doi.org/10.1186/s12916-018-1073-z
Gould IM (1999) A review of the role of antibiotic policies in the control of antibiotic resistance. J Antimicrob Chemother 43:459–465. https://doi.org/10.1093/jac/43.4.459
Stone A (2002) Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat Rev Drug Discov 1:977–985. https://doi.org/10.1038/nrd959
Álvarez-Paino M, Muñoz-Bonilla A, López-Fabal F et al (2015) Effect of glycounits on the antimicrobial properties and toxicity behavior of polymers based on quaternized DMAEMA. Biomacromolecules 16:295–303. https://doi.org/10.1021/bm5014876
Boucher HW, Talbot GH, Bradley JS et al (2009) Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases society of America. Clin Infect Dis 48:1–12. https://doi.org/10.1086/595011
Fuchs AD, Tiller JC (2006) Contact-active antimicrobial coatings derived from aqueous suspensions. Angew Chem—Int Ed 45:6759–6762. https://doi.org/10.1002/anie.200602738
Thomassin JM, Lenoir S, Riga J et al (2007) Grafting of poly[2-(tert-butylamino)ethyl methacrylate] onto polypropylene by reactive blending and antibacterial activity of the copolymer. Biomacromol 8:1171–1177. https://doi.org/10.1021/bm0611228
Ilker MF, Nüsslein K, Tew GN, Coughlin EB (2004) Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J Am Chem Soc 126:15870–15875. https://doi.org/10.1021/ja045664d
Dong C, Ye Y, Qian L et al (2014) Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose 21:1921–1932. https://doi.org/10.1007/s10570-014-0249-8
Majumdar P, Lee E, Gubbins N et al (2009) Synthesis and antimicrobial activity of quaternary ammonium-functionalized POSS (Q-POSS) and polysiloxane coatings containing Q-POSS. Polymer (Guildf) 50:1124–1133. https://doi.org/10.1016/j.polymer.2009.01.009
Nagaraja A, Puttaiahgowda YM, Kulal A et al (2019) Synthesis, characterization, and fabrication of hydrophilic antimicrobial polymer thin film coatings. Macromol Res 27:301–309. https://doi.org/10.1007/s13233-019-7040-5
Siedenbiedel F, Tiller JC (2012) Antimicrobial polymers in solution and on surfaces: overview and functional principles. Polymers (Basel) 4:46–71. https://doi.org/10.3390/polym4010046
Arora S, Yadav V, Kumar P et al (2013) Polymer based antimicrobial coatings as potential biomaterial: a review. Int J Pharm Sci Rev Res 23:279–290
Pinho AC, Piedade AP (2020) Polymeric coatings with antimicrobial activity: a short review. Polymers (Basel) 12:1–15. https://doi.org/10.3390/polym12112469
Al-Fakeh MS, Alsaedi RO (2021) Synthesis, characterization, and antimicrobial activity of CoO nanoparticles from a Co (II) complex derived from polyvinyl alcohol and aminobenzoic acid derivative. Sci World J 2021:1–11. https://doi.org/10.1155/2021/6625216
Chen Y, Cao X, Chang PR, Huneault MA (2008) Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr Polym 73:8–17. https://doi.org/10.1016/j.carbpol.2007.10.015
Suganthi S, Mohanapriya S, Raj V et al (2018) Tunable physicochemical and bactericidal activity of multicarboxylic-acids-crosslinked polyvinyl alcohol membrane for food packaging applications. ChemistrySelect 3:11167–11176. https://doi.org/10.1002/slct.201801851
Bhuvaneswari R, Karthikeyan S, Selvasekarapandian S et al (2015) Preparation and characterization of PVA complexed with amino acid, proline. Ionics (Kiel) 21:387–399. https://doi.org/10.1007/s11581-014-1206-0
Suganthi S, Vignesh S, KalyanaSundar J, Raj V (2020) Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl Water Sci 10:1–11. https://doi.org/10.1007/s13201-020-1162-y
Poverenov E, Shemesh M, Gulino A et al (2013) Durable contact active antimicrobial materials formed by a one-step covalent modification of polyvinyl alcohol, cellulose and glass surfaces. Colloids Surf B Biointerfaces 112:356–361. https://doi.org/10.1016/j.colsurfb.2013.07.032
Pencheva D, Bryaskova R, Kantardjiev T (2012) Polyvinyl alcohol/silver nanoparticles (PVA/AgNps) as a model for testing the biological activity of hybrid materials with included silver nanoparticles. Mater Sci Eng C 32:2048–2051. https://doi.org/10.1016/j.msec.2012.05.016
Hasanah AN, Elyani I, Sriwidodo et al (2015) Epichlorohydrin as crosslinking agent for synthesis of carboxymethyl cellulose sodium (Na-CMC) as pharmaceutical excipient from water hyacinth (Eichorrnia crassipes L.). Int J Chem Sci 13:1227–1237
Das K, Ray D, Bandyopadhyay NR et al (2010) Preparation and characterization of cross-linked starch/poly(vinyl alcohol) green films with low moisture absorption. Ind Eng Chem Res 49:2176–2185. https://doi.org/10.1021/ie901092n
AL-Sabagh AM, Abdeen Z (2010) Preparation and characterization of hydrogel based on poly(vinyl alcohol) cross-linked by different cross-linkers used to dry organic solvents. J Polym Environ 18:576–583. https://doi.org/10.1007/s10924-010-0200-5
Kharb R, Bansal K, Sharma AK (2012) A valuable insight into recent advances on antimicrobial activity of piperazine derivatives. Der Pharm Chem 4:2470–2488
Shinde RR, Gaikwad D, Farooqui M (2020) Synthesis and antimicrobial activity of 2-(4-(benzo[d]thiazol-5-ylsulfonyl)piperazine-1-yl)-N-substituted acetamide derivatives. J Heterocycl Chem 57:3907–3917. https://doi.org/10.1002/jhet.4099
Jalageri MD, MalgarPuttaiahgowda Y, Parambil AM, Kulal A (2019) Design of multifunctionalized piperazine polymer and its activity toward pathogenic microorganisms. J Appl Polym Sci. https://doi.org/10.1002/app.47521
Zhang M, Zeng G, Wang Y, Zhao Z (2019) MGF-Ct24E-modified piperazine polymer: a balance of antimicrobial activity and cytotoxicity. J Appl Polym Sci 136:47773. https://doi.org/10.1002/app.47773
Lan W, Zhang R, Ahmed S et al (2019) Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 113:108297. https://doi.org/10.1016/j.lwt.2019.108297
Iqbal DN, Shafiq S, Khan SM et al (2020) Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. Int J Biol Macromol 164:499–509. https://doi.org/10.1016/j.ijbiomac.2020.07.139
Yang X, Wang B, Sha D et al (2021) PVA/poly(hexamethylene guanidine)/gallic acid composite hydrogel films and their antibacterial performance. ACS Appl Polym Mater 3:3867–3877. https://doi.org/10.1021/acsapm.1c00447
Sekhavat Pour Z, Makvandi P, Ghaemy M (2015) Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol). Int J Biol Macromol 80:596–604. https://doi.org/10.1016/j.ijbiomac.2015.07.008
Chen J, Wei D, Gong W et al (2018) Hydrogen-bond assembly of poly(vinyl alcohol) and polyhexamethylene guanidine for nonleaching and transparent antimicrobial films. ACS Appl Mater Interfaces 10:37535–37543. https://doi.org/10.1021/acsami.8b14238
Olewnik-Kruszkowska E, Gierszewska M, Jakubowska E et al (2019) Antibacterial films based on PVA and PVA–chitosan modified with poly(hexamethylene guanidine). Polymers (Basel) 11:2093. https://doi.org/10.3390/polym11122093
Venkataraman S, Lee ALZ, Tan JPK et al (2019) Functional cationic derivatives of starch as antimicrobial agents. Polym Chem 10:412–423. https://doi.org/10.1039/c8py00740c
Nartop D, Demirel B, Güleç M et al (2020) Novel polymeric microspheres: synthesis, enzyme immobilization, antimutagenic activity, and antimicrobial evaluation against pathogenic microorganisms. J Biochem Mol Toxicol 34:1–13. https://doi.org/10.1002/jbt.22432
Soltani S, Akhbari K, White J (2021) Sonochemical synthesis, crystal structure and antimicrobial property of one-dimensional dinuclear coordination polymer. Zeitschrift für Anorg und Allg Chemie 647:442–447. https://doi.org/10.1002/zaac.202000268
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Ibrahim MS, Salmawi KME, Ibrahim SM (2005) Electron-beam modification of textile fabrics for hydrophilic finishing. Appl Surf Sci 241:309–320. https://doi.org/10.1016/j.apsusc.2004.07.053
Zhang M, Zeng G, Liao X, Wang Y (2019) An antibacterial and biocompatible piperazine polymer. RSC Adv 9:10135–10147. https://doi.org/10.1039/C9RA02219H
Alver Ö, Parlak C, Şenyel M (2007) FT-IR and NMR investigation of 1-phenylpiperazine: a combined experimental and theoretical study. Spectrochim Acta A Mol Biomol Spectrosc 67:793–801. https://doi.org/10.1016/j.saa.2006.08.035
Al’Abri AM, Mohamad S, Abdul Halim SN, Abu Bakar NK (2019) Development of magnetic porous coordination polymer adsorbent for the removal and preconcentration of Pb(II) from environmental water samples. Environ Sci Pollut Res 26:11410–11426. https://doi.org/10.1007/s11356-019-04467-w
Cao X, Deng P, Hu S et al (2018) Fabrication and characterization of nanoenergetic hollow spherical hexanitrostibene (HNS) derivatives. Nanomaterials 8:336. https://doi.org/10.3390/nano8050336
Król-Morkisz K, Pielichowska K (2019) Thermal decomposition of polymer nanocomposites with functionalized nanoparticles. Polymer composites with functionalized nanoparticles. Elsevier, Amsterdam, pp 405–435
Demirel B, Yaraș A, Elçiçek H (2011) Crystallization behavior of PET materials. BAÜ Fen Bil Enst Derg Cilt 13:26–35
Gadhave RV, Mahanwar PA, Gadekar PT (2019) Study of cross-linking between boric acid and different types of polyvinyl alcohol adhesive. Open J Polym Chem 9:16–26. https://doi.org/10.4236/ojpchem.2019.91002
Reena KA, Mahto V, Choubey AK (2020) Synthesis and characterization of cross-linked hydrogels using polyvinyl alcohol and polyvinyl pyrrolidone and their blend for water shut-off treatments. J Mol Liq 301:112472. https://doi.org/10.1016/j.molliq.2020.112472
Liu Z, Huang M, Li A, Yang H (2017) Flocculation and antimicrobial properties of a cationized starch. Water Res 119:57–66. https://doi.org/10.1016/j.watres.2017.04.043
Spiridon I, Anghel NC, Darie-Nita RN et al (2020) New composites based on starch/Ecoflex®/biomass wastes: mechanical, thermal, morphological and antimicrobial properties. Int J Biol Macromol 156:1435–1444. https://doi.org/10.1016/j.ijbiomac.2019.11.185
Jalageri MD, Nagaraja A, Puttaiahgowda YM (2021) Piperazine based antimicrobial polymers: a review. RSC Adv 11:15213–15230. https://doi.org/10.1039/D1RA00341K
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SK. Antimicrobial activity was performed with the help of Dr. TV and SP. The first draft of the manuscript was written by SK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanth, S., Puttaiahgowda, Y.M., Varadavenkatesan, T. et al. One-Pot Synthesis of Polyvinyl Alcohol-Piperazine Cross-Linked Polymer for Antibacterial Applications. J Polym Environ 30, 4749–4762 (2022). https://doi.org/10.1007/s10924-022-02553-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02553-8