Abstract
Cobalt(II) doped chitosan bio-composite was prepared, characterized and used to oxoborate separation from aqueous solutions. The adsorption process was carried out in a batch system. The effect of pH, temperature, time and initial boron concentration on the boron adsorption were tested. The maximum boron adsorption was reached at pH range 8.0–8.5 at room temperature and within 60 min. Calculations based on Langmuir and Freundlich models showed the heterogeneous nature of boron adsorption on Co-CTS bio-composite. On the base of research on optimization of pH and results from XRD and FTIR analysis it was found that boron adsorption by Co-CTS occurred through the co-precipitation and adsorption of B on cobalt hydroxide following the Coulomb attraction of B(OH)4 − species on positively charged sites and molecular attraction of H3BO3 through hydrogen bond with amine or hydroxyl groups of chitosan. The modelling of the thermodynamic data indicated the nonspontaneous and exothermic nature of the adsorption process. The pseudo-second-order model adequately described the boron adsorption on Co-CTS hydrogel. Desorption by means of alkaline solution at pH 12 was carried out successfully. Adsorption–desorption efficiencies in 3 cycles were almost 100%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boron dissolved in surface and ground waters is present at concentration levels of 0.3–100 mg/L and higher, anthropogenic and geothermal activity dependent [1, 2]. Boron is a significant micronutrient for plants, however, it is need only in small amounts. Its high levels are injurious and even lethal to plants. In humans, an excess of boron may lead to damage of the nervous system [3]. The boron concentration in water as well as wastewater is regulated in different countries throughout the world. The recommended level of boron in drinking and irrigating water and wastewaters discarded to the environment is 1.0 mg/L in Poland [4, 5] and in the European Union [6]. The boron concentration recommended for drinking water according to the World Health Organization guidelines is up to 2.4 mg/L [7].
There is no effective and concurrently economical method for removing boron from aqueous solution. The adsorption, membrane and hybrid processes are the most studied and applied methods. Among them the most wanted method for boron removal from water and wastewater with comparatively low boron content (up to 100 mg/L) is adsorption using inexpensive, environmental-friendly and abundant materials characterised by high affinity for orthoboric acid [8,9,10]. Many adsorbents such as fly ashes (FAs) [11,12,13], modified clays and zeolites [14, 15], some inorganic materials [16, 17], such as oxides and hydroxides of Al, Fe, Ce and Zr [18,19,20,21] and so-called layered double hydroxides (LDHs) [22, 23] have been introduced for boron removal. Nevertheless, low adsorption capacity of most of these adsorbents limits their potential applications.
Chitosan, is the second after cellulose most abundant natural biopolymer. It has excellent properties such as biocompatibility, biodegradability, non-toxicity, bacteriostatic and fungistatic properties [24]. Being cheap, environment-friendly and possessing good ability to adsorb pollutants, chitosan has attracted great interests in water and wastewater treatment. The chitosan beads, in hydrogel and freeze-dried form, have been investigated as boron adsorbents. The effects of temperature, pH, adsorbent dosage, time, and ionic strength on adsorption were examined. Adsorption of boron species onto the chitosan beads without modification occurred with physical forces and characterised with not high enough capacity [25]. The researchers’ attention has been given to the functionalized chitosan-based materials. Chitin and chitosan have improved mechanical strength and better resistance to chemical conditions after physical or chemical modification [26]. As early as 1998 chitosan resins were chemically modified by saccharides: galactose, glucose, mannose, xylose, arabinose [27], polyvinyl alcohol [28], N-methyl-d-glucamine [29, 30], glycidol [31] and propylene glycol [32].
In recent years, researchers have several other spheres of interest connecting with chitosan derivative. Accordingly, the chitosan skeleton is modified by doping with metals compounds to make sorbent with control morphologies. The choice of metal is connected with possibility of boric acid and orthoborate ions to adsorb/co-precipitate/complexate with insoluble metal hydroxide formed. Demey et al. [33, 34] noticed that chitosan-metal solution in contact (dropwise) with NaOH solution coagulates easily; thus, the formation of metal hydroxide in situ as regular beads using chitosan may be a low-cost and efficient encapsulation technique. In the paper [33], a new composite [chiNi(II)], made of chitosan and nickel(II) hydroxide, was described for the removal of boron from aqueous solutions. The maximum sorption capacity of 61.4 \({\text{mg/}}{{\text{g}}_{{\text{Ni(OH}}{{\text{)}}_2}}}\) was reached at 298 K and at pH of 8–9. Desorption studies showed the high efficiency for boron recovery using water at a pH of 12. Similarly, in the following paper [34], Demey et al. prepared [ChiFe(III)]—chitosan and iron(III) hydroxide composite beads. Adsorption experiments were carried out in a column system. The molar ratio between mass of adsorbed boron (in the five consecutive cycles) and mass of iron(III) hydroxide present in the adsorbent was 10.7 \({\text{mmo}}{{\text{l}}_{\text{B}}}{\text{/mo}}{{\text{l}}_{{\text{Fe(OH}}{{\text{)}}_3}}}\). Other metals and chitosan are also blended to form a new sorbents to remove other anions. Kamble et al. [35] applied the lanthanum incorporated chitosan (20% La-chitosan) as adsorbent for the removal of excess fluoride from drinking water. In paper [36], the fluoride removal from water by zirconium(IV) doped chitosan bio-composite was studied.
The possibility of adsorption/co-precipitation of boron on cobalt(II) hydroxide was confirmed by Turek et al. [37]. Accordingly, in this paper, cobalt(II) doped chitosan bio-composite, named Co-CTS, was the first time characterized to determine its sorption properties toward boron dissolved in water. Furthermore, for this purpose, the chemical co-precipitation process using cobalt hydroxide has been carried out.
Experimental Section
Materials
A basic standard solution of boron in the form of boric acid (H3BO3) −1 g/L (B); sodium hydroxide solution −20% (w/w) and 2 mol/L and 0.1 mol/L, hydrochloric acid solution −1 mol/L and 2 mol/L, ammonia aq. 5% (w/w), acetic acid solution, 2% (w/w). All reagents were analytically pure and supplied by POCh situated in Gliwice (Poland). Chitosan—medium molecular weight, was supplied by Aldrich.
Apparatus and Laboratory Equipment
A spectrophotometer ICP-OES Varian 710 (VARIAN); a X-ray diffractometer (Seifert XRD-3003 TT); a FT-IR spectrophotometer Nicolet 6700 (Thermo Scientific); Labmate volume pipettes (PZ HTL, Warsaw); a WU-4 universal shaker (PREMED, Warsaw); a WPE 120 electronic balance (Radwag, Radom); an analytical balance WPA 60/C (Radwag, Radom), a MILL-547 shaker with heating bath (AJL ELEKTRONIC), pH-meter Basic 20+ (CRISON); laboratory glassware and small equipment: conical flasks with ground glass joint, measuring flasks, beakers and chemical funnels.
Synthesis of Cobalt(II) Doped Chitosan Bio-composite (Co-CTS)
The procedure described in paper [38] was modified to prepare a new bio-composite as follows. Chitosan was pre-washed with distilled water (50 mL) and dried at room temperature to a constant weight. Next, 2.0 g of dry chitosan was dissolved in 2% acetic acid (98 mL) and 7.3 g of CoCl2·6H2O was added. The mixture was stirred for 24 h until complete dissolution, after which the mixture was dripped into a 20% NaOH (300 mL) using syringe with capillary forming beads (average 4 mm diameter) upon contact of gel drops with the alkaline solution. The beads stayed in NaOH solution for 24 h. The wet Co(II)—chitosan composite was washed with distilled water and rinsed to pH 7.
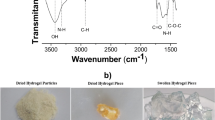
Wet gel beads were divided into three portions. The first was left under water as wet hydrogel beads. The second was dried, at 100 °С for 12 h in a drier while the third portion of beads was freeze-dried in lyophilizing cabinet to remove the water from the pore structure. The dry beads were then powdered in agate mortar and sieved to a constant size <0.45 mm. The Co-CTS powder was analyzed by X-ray diffraction (XRD) analysis to determine the structures resulting from the formation process of bio-composite. The formation of the Co-CTS films was studied by IR spectroscopy, with a Thermo Scientific Nicolet 6700 FT-IR spectrophotometer using attenuated total reflectance (ATR method). The content of total cobalt in bio-composite was determined by ICP OES method after dissolving the wet gel in 0.1 M HCl solution.
Methods
A series of the batch-mode adsorption studies were conducted to evaluate the effects of the initial boron concentration, pH level, temperature, and time on boron adsorption. The experiments were carried out with 1.0 g of gel and 0.1 g of powder and 20 mL of boric acid solution at different boron concentration (2–200 mg/L) at optimum pH (adjusted by addition of 1 M HCl or 0.1 M NaOH solution) at 20–65 °C temperature in 100-mL well-stoppered conical flask until the adsorption reached equilibrium. Then, boron solution and Co-CTS beads were shaken at 60 rpm mixing rate in a mechanical shaker. At the end of the experiment, the suspension was filtered through medium paper filters. For kinetic studies 4.0 g of chitosan beads was added to a capped volumetric flask containing 100 mL of boric acid solution (boron concentration of 20 mg/L) at pH of 8.5, then aliquots were taken at appropriate time intervals after shaking on mechanical shaker and filtered as above. Adsorption test as a function of pH were conducted by shaking 1 g wet/0.1 g dry beads with 20 mL of boric acid solution at boron concentration of 20 mg/L at different pH (adjusted by addition of 1 M HCl or 0.1 M NaOH solution) for 1 h. The filtrate was analyzed for boron and cobalt concentration by the ICP-OES method. Each adsorption experiment was repeated three times in order to have average values. The reproducibility was found to be within ±3%.
The boron adsorption coefficients (the adsorption efficiency, A [%], and the adsorption capacity, q [mg/g]) were calculated from the experimental data in each sample using the following equations:
where C 0 and C are the initial and final concentration of boron in the solution [mg/L], respectively, V 0 is the volume of the B solution [L] and m is the dry mass of the adsorbent [g].
For the mechanism studies of adsorption, the behaviour of boron and cobalt species together present in solution during pH changes was examined. For this purpose, the binary solution of cobalt(II) at concentration of 2000 mg/L and boron(III) at concentration of 20 mg/L was made. Then, the pH values of the solution was adjusted at 4–11 by 0.1 M HCl and 0.1 M NaOH solutions. After adsorption equilibrium reached, the mixture was separated by centrifugation and filtration (if the suspended solids was present) and the concentrations of boron and cobalt in filtrates were determined by the ICP-OES method.
Furthermore the point of zero charge (pHpzc) of a new adsorbent was determined according to Balistrieri and Murray method [39]. To a series of well-stoppered 100 mL polyethylene bottles containing 40 mL of 0.01 M KNO3 solution, different volumes of either 0.1 M HCl or 0.1 M NaOH solution were added to obtain pH values from 5 to 10. The total volume of solution in each bottle was made up to 50 mL by addition of demineralized water. After 2 h of equilibration the pH values (pHi) were noted and 0.2 g of Co-CTS sample to each solution was added. After 72 h of equilibration with discontinuous shaking, the pH value of the supernatant liquid in each bottle was noted (pHf). The difference between pHi and pHf (ΔpH) was then plotted against pHi. The solution pH at which pH 0 means “the pHpzc” of the sample [40]. Similarly, the pHpzc of Co-CTS in the contact with boric acid solution of 20 mg/L (B) was determined.
The effect of pH on boron adsorption on Co-CTS was investigated using boron stock solution (5 and 20 mg/L) with pHs varied from 4 to 11 and amount of 1 and 0.1 g of hydrogel and powder Co-CTS, respectively. The samples were shaken for 1 h at the room temperature (25 °C) to reach equilibrium. The boron uptake on Co-CTS was measured using ICP-OES method.
For regeneration of Co-CTS alkaline water was used. The experiment was carried out with 1.0 g of gel placed in 50-mL well-stoppered conical flask. 10 mL of sodium hydroxide solution at concentration of 0.001 or 0.01 mol/L was added to the beads and shaken at 60 rpm mixing rate in a mechanical shaker within 10 min at room temperature. Finally, the suspension was filtered through medium paper filters and boron and cobalt concentrations were determined in filtrate.
Results and Discussion
Characteristic of Co-CTS
The X-ray diffraction experiments were performed on a Seifert Analytical X-ray diffractometer operating at 30 mA and 40 kV, which was equipped with a vertical goniometer and Eulerian cradle. The radiation wavelength (λ, CuKα) was 1.54178 Å. The grazing incidence X-ray diffraction (GIXD) patterns were recorded over a 2θ range from 10° to 80° with a 0.05° step size for the incident angle (α = 0.25°). Then the region between 20° and 60° was selected to the measurement with a 0.05° step size the incident angle (α = 0.25°), the measurement was three time repeated. As seen from Fig. 1a the signals from cobalt hydroxide and cobalt chloride were detected. It was reported that the broad peaks between 7° and 10° and near 20 °C are characteristic for the chitosan. In our case the intensities of the signals were very low, it could be caused by presence of amount of water and amorphous nature of chitosan. The broadness of reflections, low peak intensities were characteristic of a poorly crystalline phases presence in the samples.
IR spectroscopy, with a FT-IR spectrophotometer (ATR method) confirmed the bonding between chitosan and cobalt (Fig. 1b). The bands at 3629 and 3367 cm−1 are typical for stretching vibrations of O-H and N-H bonds. The IR band at 1644 cm−1 can be assigned to deformation vibrations of C=O bonds. Absorption bands recorded in the region of 1073–1378 cm−1 are characteristic for stretching vibrations of C–N and C–O bonds. The reaction of chitosan with cobalt ions leads to the formation of structure with Co–O and O–H bonds which are visible at 561 cm−1.
The total content of cobalt in bio-composite determined by ICP OES method amounted 43.2 ± 0.5 mg/g.
Effect of pH and Mechanism of Boron Sorption
pH has a very significant effect in studying of boron removal from aqueous solution (as seen from Fig. 2). The speciation of boron in aqueous solutions is known long ago. At the boron concentration less than 250 mg/L, H3BO3 and B(OH)4 − species mainly exist, however, the fraction of boric acid decreases while fraction of borate anion increases when pH changes from acidic to basic reaction. The distribution of boron species is 50% of H3BO3 and 50% of B(OH)4 − at pH 9.2 [32]. In this study boron adsorption also depended on distribution of cobalt species, especially on the precipitation of cobalt hydroxide. As apparent from [41], the Co2+ species remain dominant until pH of about 8 and then decrease sharply, to Co(OH)2 species advantage, which exhibit a maximum at pH 11 and undergo precipitation as Co(OH)2(s).
Separation of boron from the bivalent solution Co-B species is shown in Fig. 3. The adsorption/co-precipitation of boron probably on Co(OH)2(s) is maximum around pH 8.6 and decreases from 35 to 15% at pH above 11 due to solubilization of cobalt hydroxide. Similar pH dependence occurs during boron separation using chitosan doped with cobalt(II). Boron adsorption on Co-CTS as a function of pH is shown in Fig. 2. The maximum adsorption capacity was found at pH 8.0 and pH 8.5 for initial boron concentrations 5 and 20 mg/L, respectively. By decreasing the pH from 8 to 4, boron adsorption capacity of Co-CTS first decreased to achieve a minimum at pH 7 and next increased slightly to stabilize the level of boron capacity. Demey et al. [33] reported that when the pH is below the pKa value of chitosan (6.3–6.8), amine groups are protonated and work as the sites of forming complex with boric acid present in low pHs.
However, at more alkaline pH (>9.0) boron adsorption of Co-CTS was found to be decreased by almost 40–50%. At pH 11 there is not adsorption of boron on Co-CTS. The decrease of boron uptake at strongly alkaline pH conditions may be due to electrostatic repulsion of orthoborate to negatively charged surface chitosan, besides competition for active cationic site bindings by excessive presence of hydroxyl anions. These results are in good agreement with the research on adsorption/co-precipitation of boron on Co(OH)2(s) described above. It means that the precipitation of cobalt hydroxide during formation of modified chitosan hydrogels is the basis of new biocomposite stability and adsorption capacity towards boron.
Point of zero charge (pHpzc) for new adsorbent was calculated through the pHi and pHf values derived from solid addition technique. The point at which the ΔpH (pHi–pHf) equals zero would be considered as pHpzc. From Fig. 4, the point of zero charge values for Co-CTS hydrogel (H) and thermal-dried (T) were found to be 8.48 and 8.36, respectively. Since point of zero charge (pHpzc) for Co-CTS hydrogel is around 8.5 and 9.0 in the presence of H3BO3 solution, respectively, surface of adsorbent is positively charged till pH < pHpzc. The anionic species would thus have higher stronger interaction with Co-CTS and have higher uptake. At pH > 8.5 the surface charge of Co-CTS hydrogel is negative, favoring the cations adsorption. Given the fact that the maximum boron adsorption was reached at pH 8–8.5, all experiments were conducted at this pH range.
Literature reveals chitosan-derivative sorbents were used for removal of cations, due to free electron lone pairs in O and N-atoms of chitosan linkage. Thus, metals like cobalt(II) get catches at primary –NH2 and OH groups of chitosan due to electrostatic attraction and precipitate as hydroxide at pH upon 8. This metal doped chitosan cationic site is vital in borate anion adsorption mechanism. Besides, borate adsorption efficiency decreases above pH > 10. This desorption rate can be interpreted, first, as hydroxide anions competent to borate on coordinated sites of cobalt chitosan composite via ligand-exchange mechanism and second due to dissolving of Co(OH)2 in advantage to Co(OH)3 −. These results indicated that borates co-precipitated and together with cobalt(II) and hydroxyl groups formed stable complex. A new Co-CTS is able to lower oxoborate level and can be applied to manifold deboronation of drinking water.
Equilibrium Studies
The adsorption equilibrium was studied at a temperature of 25 °C, and pH 8.5, and in the initial boron concentration range of 5–200 mg/L while the dose of adsorbent was held constant at 1 g of hydrogel and 0.1 g of powder per 20 mL of solution and contact time of 1 h. As it can be seen from Fig. 5, the adsorption isotherms were regular, positive and convex to the concentration axis for Co-CTS hydrogel (H), thermal-dried (T) and lyophilized (L). The curves achieved a saturation plateau at high boron concentration. The initial slopes are not very steep, indicating that the affinity of the Co-CTS for boron is not very strong. Maximum adsorption capacity q amounted to 2.5, 1.6 and 1.2 mg/g for Co-CTS (H), (T) and (L), respectively.
Langmuir and Freundlich models were tested [42, 43]. The Langmuir model assumes that the adsorption occurs in surface sites where the energy is equal in each size while the Freundlich model allows for several kinds of adsorption sites in the solid, each having a different energy of adsorption. The Langmuir equation is represented by Eq. 3:
where q m and B are the Langmuir parameters, q m is the adsorption capacity [mg/g], expressed as the maximum amount of boron that can be adsorbed by the adsorbent as a monolayer and B is an equilibrium constant that corresponds to the adsorption energy [L/mg]. The Freundlich isotherm is given by Eq. 4:
where the parameters K [mg/g] and n correspond to the relative adsorption capacity and the adsorption intensity of the adsorbent, respectively.
Both Langmuir and Freundlich data fitting was done by linearization of Eqs. 3 and 4 as shown by Eqs. 5 and 6, respectively:
Plotting 1/q versus 1/C gives a curve with the slope 1/(q m × B) and intersection 1/q m . Plotting log q versus log C results in a straight line with the slope 1/n and intersection log K.
The essential characteristics of a Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor R L which is defined by Eq. 7:
According to Hall et al. [44], the parameter R L indicates the shape of the isotherm accordingly: R L > 1, unfavorable; R L = 1, linear; 0 < R L < 1, favorable; and R L = 0, irreversible. Similarly, the fitness of using the Freundlich equation to describe the adsorption can be assessed by the constant n. If 1 < n < 10, the Freundlich equation is adequate for use [45].
The Langmuir equation fitted the experimental data worse than the Freundlich equation because the correlation coefficients (R2) equal 0.9768, 0.9611 and 0.9613 for Co-CTS (H), (T) and (L), respectively while Langmuir correlation coefficients are below 0.9000 (Table 1). The Freundlich model usually corresponds to the adsorption process on heterogeneous surfaces. Furthermore, the fitness of using the Freundlich equation to describe the adsorption can be assessed by the constant n. If 1 < n < 10, the Freundlich equation is adequate for use [45]. As we can see in Table 1, the parameter n contains in this range for each kind of Co-CTS adsorbent. The n value greater than 1 means an S-type isotherm and also corresponds to physical adsorption [46]. K value was calculated to be 0.020, 0.048 and 0.028 mg/g at 25 °C for Co-CTS (H), (T) and (L) Co-CTS, respectively.
Similarly, Bursali et al. [25] found that the Freundlich equation provided better conformity than the Langmuir equation: experimental data of boron adsorption on chitosan beads confirmed to the Freundlich equation on the basis of the formation of multilayer adsorption. Differently, the Langmuir equation gave the best fit of the experimental data for composite [chiNi(II)], made of chitosan and nickel(II) by Demey et al. [33].
Although the Langmuir model is less suitable than the Freundlich model for the representation of the adsorption data, the calculated values qm (2.3, 1.5, 1.2 mg/g) are in good agreement with the experimental q given above. The RL values calculated with Eq. 7 indicate that boron adsorption on Co-CTS is favorable at 25 °C for the boron concentration range of 5–200 mg/L (Table 1). Comparing of the maximum adsorption capacities of chitosan found in the literature, Co-CTS composites have a capacity of the same order of magnitude as other adsorbents, e.g. Bursali et al. [25] calculated the adsorption capacity of boron on chitosan beads about 2.5 mg/g at 25 °C. Although composite [chiNi(II)], made of chitosan and nickel(II) hydroxide [33] and oxide/hydroxide-based nanoparticles (NanoFe) [47] present better q, they have a great disadvantages. [chiNi(II)] composite cannot be used in drinking water treatment because of nickel and its compounds are known as allergens. When applying the NanoFe, post-treatment requires the elimination of nanoparticles as colloidal precipitates using additional processes such as coagulation, sedimentation and filtration.
Finally, it was found that pH changed very negligibly after adsorption process (pHfinal = 8.0–8.8) and no precipitate was detected in the supernatant. The cobalt concentration determined in supernatant was below 1 mg/1 L.
Adsorption Kinetics
The variation of the boron adsorption with respect to time, t, at the adsorbent dosage of 4.0 g/100 mL, an initial boron concentration of 5 and 20 mg/L, an initial pH value of 8.5 and temperature of 25 °C is shown in Fig. 6. Generally, the adsorption was rapidly increasing until maximum, t = 120 min and t = 30 min, for initial boron concentration 5 and 20 mg/L, respectively. The equilibrium was reached after 480 min for both initial concentration of boron. In order to explain the mechanism involved in the adsorption process, the three kinetic models: the Lagergren pseudo-first and pseudo-second order, the Elovich equation, and the parabolic diffusion model were tested [48, 49]. The pseudo-first-order model is given by the Eq. 8:
where q t and q e are the amounts of boron adsorbed [mg/g] at any time t and at the equilibrium time, respectively, and k 1 is the pseudo-first-order rate constant for the sorption process [1/h].
The pseudo-second-order kinetics is expressed by the Eq. 9:
where k 2 is the pseudo-second-order rate constant [g/(mg h)].
The overall kinetics of the sorption from solutions may be governed by the diffusional processes as well as by the kinetics of the surface chemical reaction. In diffusion studies, the rate is often expressed in terms of the square root time (Eq. 10):
where k p is the intraparticle diffusion rate constant [mg/(g/(h)1/2)].
The plots of log (q e −q t ) versus t, t/q t versus t and q t versus t 1/2 obtained from above models checked statistically and graphically should be a straight line with the slope −k 1/2.303 and intersection log q e , the slope 1/q e and intersection \({\text{1/}}{k_{\text{2}}}q_{e}^{{\text{2}}}\) respectively, and k p as a slope of the plot of q t versus t 1/2 if the sorption process fits to the related equation. Moreover constant k 2 is used to calculate the initial sorption rate at t = 0, r, which is defined by Eq. 11:
where r is the initial sorption rate [mg/(g h)].
The resulting parameters of these models calculated and the correlation coefficients R2 are listed in Table 2, where the experimental value of qexp is also presented. The results show that the pseudo-first-order rate kinetic model and the intraparticle diffusion model are not applicable for the presented process due to the lower correlation coefficients and disagreement between the calculated values q1 and the experimental ones: qexp = 0.367 mg/g (initial B conc. 5 mg/L) and 0.495 mg/g (initial B conc. 20 mg/L). For the pseudo-second-order rate kinetic model, the coefficient \({\text{R}}_{{\text{2}}}^{{\text{2}}}\) was 0.9998 and 1.000, the calculated values q2 amounted 0.353 mg/g for initial B conc. 5 mg/L and 0.496 mg/g for initial B conc. 20 mg/L and agreed with the experimental ones given above. This means that the pseudo-second-order model very good describes the boron adsorption on wet Co-CTS gel beads.
Influence of Temperature
In order to understand the chemical thermodynamics of the adsorption process, the temperature influence on the boron adsorption on Co-CTS was determined out at pH 8.5 and a temperature of 25, 45 and 65 °C for an initial boron concentration of 20 mg/L, a shaking time of 1 h and a adsorbent dose of 1 g/25 mL (wet Co-CTS hydrogel beads) and 0.1 g/25 mL (dry Co-CTS powder). The variation of the boron adsorption with respect to temperature, T, is shown in Fig. 7. Generally, the boron adsorption decreased with an increase of temperature from 25 to 65 °C both Co-CTS hydrogel (H) and Co-CTS thermal-dried (T).
The thermodynamic parameters: molar free energy change, ∆G ɵ, standard enthalpy change, ∆H ɵ, and standard entropy change, ∆S ɵ, were calculated [50]. ∆G ɵ is related to the equilibrium constant (K c ) and calculated from the Eq. 12:
where R is the gas constant [8.314 J/(mol K)], T is the temperature [K] and K c is the equilibrium constant. The K c values were estimated as:
Each K c value was the average of all experimental values (C 0 and C) obtained at constant temperature that the adsorption experiments were carried on.
∆H ɵ and ∆S ɵ can be estimated using the following equation:
Plotting ln K c versus 1/T produces a straight line with the slope −∆H ɵ/R and intersection ∆S ɵ/R.
Standard entropy and enthalpy changes of the boron adsorption were calculated as, ∆S ɵ = −0.17 kJ/(mol K) and ∆H ɵ = −45.36 kJ/mol, ∆Sɵ = −0.27 kJ/(mol K) and ∆Hɵ = −74.31 kJ/mol, for Co-CTS (H) and Co-CTS (T), respectively (Table 3). The negative value of ∆S ɵ indicated a decreased randomness at the solid–solution interface during the adsorption of boron on the Co-CTS. The negative value of ∆H ɵ showed the exothermic nature of the process. As we can see from Table 3 and Fig. 7, boron adsorption and K c (equilibrium constant) values decreased with an increase of temperature. In contrast, the ∆G ɵ values increased when the temperature increased. The positive values of ∆G ɵ at 25, 45 and 65 °C implied that the adsorption of boron on Co-CTS was not spontaneous. The negative ∆S ɵ value corresponds to a decrease in the degree of freedom of the adsorbed species.
Desorption Tests
NaOH (0.001 and 0.01 mol/L) solution was used as eluent for desorbing boron from Co-CTS. The boron desorption coefficient (the desorption percentage, D [%]) was calculated from the experimental data using the following equation:
where q S is the boron adsorption capacity and q D is the amount of boron desorption per adsorbent mass [mg/g]. It was found that 47.6 ± 4.6 and 98.5 ± 0.9% of boron had been eluted within 10 min of contact with 0.001 and 0.01 mol/L of NaOH solution, respectively.
To determine the reusability of Co-CTS, adsorption–desorption cycles were repeated three times. The quantitative desorption yield in first, second and third cycle was confirmed. Adsorption–desorption efficiency, ES−D [%], was calculated using the following relationship:
where q n is the total adsorption capacity in the first cycle and q n+1 is the total adsorption capacity in the next cycle [mg/g]. The ES−D value was determined 99.7 ± 0.6, 99.7 ± 4.6 and 99.2 ± 4.8% in the first, second and third cycle, respectively. The result of 100% adsorption–desorption efficiency, means that no decrease in the boron adsorption and desorption occurred obtained at 2nd and 3rd cycle.
Conclusions
A new bio-composite, cobalt(II) doped chitosan, was prepared, characterized and used to boric acid/oxoborate separation from aqueous solutions by adsorption process in batch system.
The XRD and FTIR analyses showed that a hydrogel beads of chitosan containing cobalt hydroxide was obtained. Furthermore, the experimental results confirmed that new bio-composite had an affinity to boron, however the boric acid sorption on Co-CTS was significantly affected by pH of solution and the contact time between phases. The Freundlich isotherm fitted better the experimental data than the Langmuir model. It indicates the heterogeneous nature of boron adsorption on Co-CTS bio-composite. The parameters of Freundlich equation corresponds to multilayer adsorption and characterised boron adsorption on Co-CTS means as S-type isotherm.
It was concluded that B(OH)4 − species were adsorbed at optimum values of pH 8.0–8.5 through the Coulomb attraction and B co-precipitation/adsorption by Co(OH)2. The kinetic study revealed that the pseudo-second-order mechanism was the rate determining step boron adsorption on Co-CTS. Optimum time of adsorption was determined as 1 h. The thermodynamic experiments showed that boron adsorption decreased with an increase in temperature of the solution (∆Hɵ < 0) and the process was nonspontaneous (∆Gɵ > 0).
Maximum adsorption capacity was 2.5, 1.6 and 1.2 mg/g for the Co-CTS hydrogel, thermal-dried and lyophilized, respectively. Desorption by means of alkaline solution at pH 12 was carried out successfully (D = 98–101%) with no loss of cobalt ions.
The new adsorbent Co-CTS can be interesting in surface water treatment to remove boron because of non-toxicity, bacteriostatic and fungistatic properties. The big advantage is the ease of Co-CTS regeneration with 0.01 M NaOH and nearly 100% efficiency ratio of adsorption–desorption.
References
WHO (1998) Environmental health criteria 204 for Boron. International Programme on Chemical Safety. WHO, Geneva, ISBN 92 4 1572043
Hilal N, Kim GJ, Somerfield C (2011) Desalination 273:23
Bobrowska-Grzesik E, Ciba J, Grossman A, Kluczka J, Trojanowska J, Zołotajkin M (2013) Chemical Elements Compendium. 2 Theta, Cesky Tesin
Bills and Acts Bulletin. (2002). No. 203, item 203
Bills and Acts Bulletin. (1987). No. 42, item 248
EU Drinking Water Directive, 1998. (98/93/EC)
Guidelines for drinking-water quality, 4th edn. World Health Organization (WHO), 2011, ISBN 978 92 4 154815 1
Chieng HJ, Chong MF (2012) Global J Environ Sci Technol 2:11
Nasef MM, Nallappan M, Ujang Z (2014) React Funct Polym 85:54
Guan Z, Lv J, Bai P, Guo X (2016) Desalination 383: 29
Ahmaruzzaman M, Gupta VK (2011) Ind Eng Chem Res 50(24):13589
Yüksel S, Yürüm Y (2010) Sep Sci Technol 45:105
Kluczka J, Trojanowska J, Zołotajkin M (2015) Desalin Water Treat 54(7):1839
Karahan S, Yurdakoç M, Seki Y, Yurdakoç K (2006) J Colloid Interface Sci 293:36
Kluczka J, Korolewicz T, Zołotajkin M, Simka W, Raczek M (2013) Environ Technol 34:1369
Ferreira OP, de Moraes SG, Durán N, Cornejo L, Alves OL (2006) Chemosphere 62(1)
Kavak D (2009) J Hazard Mater 163:308
Demetriou A, Pashalidis I, Nicolaides AV, Kumke MU (2013) Desalin Water Treat 51:6130
Demetriou A, Pashalidis J (2012) Desalin Water Treat 37(1–3):315
Öztürk N, Kavak D (2008) Desalination 223: 106
Kluczka J (2015) Int J Environ Res 9(2):711
Kentjono L, Liu JC, Chang WC, Irawan C (2010) Desalination 262(1–3):280
Koilraj P, Srinivasan K (2011) Ind Eng Chem Res 50(11):6943
Ravi Kumar MNV (2000) React Funct Polym, 46:1
Bursali EA, Seki Y, Seyhan S, Delener M, Yurdakoc M (2011) J Appl Polym Sci 122(1):657
Xiao M, Hu J, Liu W, Nie F (2014) Adv Mater Res 1048:373
Matsumoto M, Matsui T, Kondo K (1999) J Chem Eng Jpn 32(2):190
Harada A, Takagi T, Kataoka S, Yamamoto T, Endo A (2011) Adsorption 17:171
Wei YT, Zheng YM, Chen JP (2011) Water Res 45:2297
Sabarudin A, Oshita K, Oshima M, Motomizu S (2005) Talanta 66(1):136
Kluczka J, Korolewicz T, Zołotajkin M, Adamek J (2015) Water Res Ind 11:46
Gazi M, Shahmohammadi S (2012) React Funct Polym 72:680
Demey H, Vincent T, Ruiz M, Nogueras M, Sastre AM, Guibal E (2014) Chem Eng J 244:576
Demey H, Vincent T, Ruiz M, Nogueras M, Sastre AM, Guibal E (2014) Chem Eng J 254:463
Kamble SP, Jagtap S, Labhsetwar NK, Thakare D, Godfrey S, Devotta S, Rayalu SS (2007) Chem Eng J 129:173
Dongrel R, Ghugal DN, Meshram JS, Ramteke DS (2012) Afr J Environ Sci Technol 6(2):130
Turek M, Dydo P, Trojanowska J, Campen A (2007) Desalination 205:192–199
Krzesińska M, Tórz A, Zachariasz J, Muszyński J, Socha J, Marcinkowski A (2007) Green Chem 9:842
Balistrieri LS, Murray JW (1981) Am J Sci 281:788
Mohapatra M, Rout K, Mohapatra BK, Anand S (2009) J Hazard Mater 166:1506
Yüzer H, Kara M, Sabah E, Çelik MS (2008) J Hazard Mater 151:33
Paderewski ML (1999) Adsorption processes in chemical engineering. WNT, Warszawa (in Polish)
Duong DD (1998) Adsorption analysis equilibria and kinetics. Imperial College Press, London
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Ind Eng Chem Fundam 5:212
Treybal RE (1980) Mass transfer operations. McGraw Hill, New York
Giles CH, D’Silva AP, Easton IA (1974) J Colloid Interface Sci 47:766
Zelmanov G, Semiat R (2014) Desalination 333:107
Weber WJ, Morris JC (1963) J Sanit Eng Div ASCE 89:31
Ho YS, McKay G (1998) Chem Eng J 70:115
Öztürk N, Kavak D (2005) J Hazard Mater 127(1–3):81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kluczka, J., Tórz, A., Łącka, D. et al. Boron Removal by Adsorption on Cobalt(II) Doped Chitosan Bio-composite. J Polym Environ 26, 2039–2048 (2018). https://doi.org/10.1007/s10924-017-1099-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1099-x