Abstract
Chemical modification of potato starch with citronellyl methacrylate monomer, characterization of obtained materials and its physicochemical properties have been presented. The chemical modification of potato starch under the grafting process with citronellyl methacrylate led to the preparation of novel, amphiphilic materials where from 0.3 to ca. 1.6 hydroxyl groups per glycoside unit were replaced by poly(citronellyl methacrylate) chains. The grafting of poly(citronellyl methacrylate) chains onto starch backbone caused considerably changes in the morphology, polarity, solubility, chemical stability, moisture absorbance, gelatinization properties, thermal stability and decomposition mechanism of the prepared copolymers as compared to native potato starch. The influence of the grafting percent on the above mentioned properties as well as on the pyrolysis mechanism was discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citronellol is a monoterpenoid, primary fragrant alcohol with sweet, agreeable, comparable with rose odour. It is found in the nature in some essential oils such as citronella oil which is raised from the stems and leaves of different species of Cymbopogon. The citronellol is also found in rose oil, Eucalyptus citriodora, Boronia citriodora and Pelargonium geraniums oil. It can be obtained by the extraction or distillation from the plants or by the hydrogenation process of nerol or geraniol. According to the literature survey, this alcohol is commonly applied in the perfumery and cosmetic industries, it is added to candles, soups or incense as a source of flavor. Also, citronellol can be applied as a plant-based insect (mosquito) repellent, mite or pets attractant or antifungal agent. Due to the presence of the primary hydroxyl groups in it structure, it is used as a chemical for the production of rose oxide under the cycle of transformations including it photooxygenation to allyl hydroperoxide, it reduction with sodium sulfite to diol and finally diol ring-closure in the presence of sulfuric acid. Also, citronellol is used for the preparation of hydroxydihydrocitronellol and hydroxydihydrocitronellal [1,2,3,4,5,6,7,8,9]. It is useful reagent in the preparation of the aroma monoesters, diesters or tetraesters of acids and acid anhydrides. Monoesters of citronellol found their place as a fragrance or flavors added to many detergents, perfumes, medical products or laundry liquids. However, the diesters or tetraesters can be potential flavor compounds for different products or plasticizers for polymers [9,10,11,12,13,14,15,16,17,18]. Citronellol is also suitable reagent for the synthesis of it methacrylate ester under the lipase catalysed transestrification reaction using the following acylating agents: methyl methacrylate, vinyl methacrylate and 2,3-butanedione monooxime methacrylate [19]. The synthesis of citronellyl methacrylate with high yield can be performed under the amine catalysed reaction of citronellol with methacryloyl chloride according to Ref [20]. which is presented in this paper. This compound due to the presence of polymerizable double–double bonds in it structure can be useful monomer for the preparation of polymers and copolymers.
This paper presents the utilization of citronellyl methacrylate prepared under the esterification process of naturally occurring terpene alcohol and methacryoyl chloride in the presence of triethylamine as a catalyst in the synthesis of copolymers with potato starch and the evaluation of the properties of the obtained materials. The copolymers were prepared under the free-radical graft copolymerization process of gelatinized potato starch with citronellyl methacrylate ester according to the procedure presented elsewhere [21,22,23]. By using this procedure, the series of novel copolymers which differ in its grafting parameters have been obtained. Their structure was confirmed based on the spectroscopic methods. The evaluation of the influence of the grafting parameters on the swelling, moisture absorbance, gelatinization, chemical stability in acid, alkaline and buffer solutions, thermal stability and its decomposition mechanism has been performed.
Experimental
Materials
Potato starch (amylopectin/amylose content: 83/17, purity 98%) was isolated from potato flour containing 12% water (Melvit S.A., Poland) according to the procedure given in Ref [24]. Methacryloyl chloride (97%), triethyloamine (99%) were delivered by Sigma-Aldrich. Citronellol (95%) was from Fluka, Switzerland. Chloroform, methanol, tetrahydrofurane, potassium persulfate and silica gel were obtained from Merck, Germany. Hydrochloric acid, sodium carbonate and buffer solutions were from POCh, Gliwice, Poland.
Synthesis of Monomer
The monomer was prepared and purified according to the procedure described elsewhere [20]. The esterification reaction of citronellol (0.1 mol) by methacryoyl chloride (0.12 mol) in the presence of a catalyst: triethylamine (0.12 mol) was performed, Fig. 1. As a solvent, chloroform (150 mL) was applied. The reaction at the temperature of 5 °C for 1h and then at room temperature for the next 1h was carried. The raw product was purified by washing with 10% HCl, 1% Na2CO3 and distilled water. After drying over MgSO4, the organic layer was cleaned using chromatographic colum filled with silica gel. As an eluent, chloroform was applied. The above reaction conditions allowed obtaining liquid, colorless monomer with high yield (95%). The chemical structure of the monomer was confirmed based on the spectroscopic methods.
On the ATR-FTIR spectrum gathered for the monomer, the presence of the absorption signals characteristic for the out of plane deformation vibrations for =C–H (755–1004 cm−1), the stretching vibrations for C=O (1035–1294 cm−1), the deformation vibrations for C–H (1375–1446 cm−1), the stretching vibrations for C=C (1633 and 1672 cm−1), the stretching vibrations for C=O (1716 cm−1), the stretching vibrations for C–H (2850–1958 cm−1) and the stretching vibrations for =C–H (3020 cm−1) has been identified [25]. The 1HNMR spectrum shows the resonance signals responsible for the protons assigned to methacrylate carbon–carbon double bonds at 6.1 and at 5.5 ppm, for the proton attached to the citronellyl carbon–carbon double bond at 5.1 ppm, for the protons at CH2–O group at 4.2 ppm, for the protons at CH3 group near the methacrylate carbon–carbon double bond and for the CH2 group in the citronellyl skeleton at 2.0 ppm, for the protons at CH3 groups in the citronellyl skeleton at 1.6, 1.7 and 0.9 ppm, for the protons at CH2 groups and at CH group in the citronellyl skeleton at 1.2, 1.3 and 1.4 ppm. All resonance signals characteristic for carbons occurring in the monomer skeleton were also clearly visible from the presented 13CNMR spectrum: at 167.6 ppm (C=O), at 136.6 ppm (=C<), at 131.4 (=C<), at 125.2 ppm (=CH), at 124.7 ppm (=CH2), at 63.3 ppm (CH2), at 37.1 ppm (CH2), at 35.5 ppm (CH2), at 29.6 ppm (CH), at 25.5 ppm (CH3), at 19.6 ppm (CH3), at 18.4 ppm (CH3), at 17.7 ppm (CH3) [26], Fig. 2.
Graft Copolymerization
The graft copolymerization process of potato starch with citronellyl methacrylate was performed according to the procedure described in Refs [21,22,23]. applying the following optimal reactions conditions: the temperature of 80 °C, reaction time of 2 h, initiator concentration (2 wt%) and the different potato starch to monomer ratio (from 1:0.25 up to 1:2.0).
The grafting parameters: grafting efficiency (%GE), grafting percent (%G) and homopolymer percent (%H) were counted based on the following equations [27,28,29]:
where m2 is the mass of homopolymer, m3 is the mass of grafted polymer
where m1 is the mass of starch, m3 is the mass of grafted polymer
where m2 is the mass of homopolymer, m0 is the mass of monomer charged.
Characterization of Copolymers
FTIR spectra of copolymers by applying the Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) technique on a Bruker Tensor 27 instrument, Germany equipped with diamond crystal, over a resolution of 4 cm−1 and wavenumber region of 600–4000 cm−1 have been collected.
Cross-polarisation magic angle spinning (13C CP/MAS NMR) spectra using a Bruker Avance 300 MSL instrument, Germany over the resonance frequency of 75.5 MHz have been recorded.
Scanning electron microscopy (SEM) microphotografts using a high resolution, low vacuum SEM/FIB, the Quanta 3D FEG system, FEI company with a magnification of 3000× and 8000× have been done.
Water, ethanol, butanol, toluene, hexane and CCl4 were applied as solvents in order to evaluate the swellability coefficients (B) of the obtained materials. The B values were calculated by equilibrium swelling in chosen solvents, using the centrifugation method based on a below equation [30]:
where V1 is the volume of the copolymer after swelling, V0 is the volume of the dried copolymer.
Percent moisture absorbance (%M) was evaluated according to the following equation [31]:
m2 is the final mass of the sample (after moisture absorbance study), m1 is the initial mass of the sample (before moisture absorbance study).
In order to evaluate %M, the ca. 100 mg of the dried sample was placed in an exsiccator and exposured to the water vapour at 25 °C for 24 h and then weighted.
To evaluate the gelatinization properties of the copolymers, ca. 3 mg of the copolymer and ca. 6 mg of water were mixted and put away at room temperature for 24 h to equlibrate. Then, the samples were heated in close aluminium pans between the temperature of 20–100 °C with a heating rate of 10 K/min under argon atmosphere (flow rate 20 mL/min) using DSC Phoenix 204 instrument, Netzsch, Germany [21].
The chemical resistance study of the obtained copolymers were evaluated in 1 M HCl, 1 M NaOH and buffers with pH 5, 7, 9. The percent mass loss of the copolmers in the chosen solutions was calculated based on the below equation [32]:
where m1 is the initial mass of the dried sample, m2 is the final mass of the sample (after chemical resistance studies).
The thermal properties of the copolymers by applying STA F1 Jupiter instrument Netzsch, Germany were evaluated. The copolymers were heated in open Al2O3 with a heating rate of 10 K/min from 40 °C up to 1000 °C under helium atmosphere (a flow rate of 40 mL/min) and a sample mass ca. 10 mg.
The decomposition mechanism of copolymers using FTIR spectroscopy coupled with STA F1 Jupiter instrument was evaluated. The gaseous FTIR spectra over the wavenymber range of 600–4000 cm−1 and a resolution of 4 cm−1 were gathered.
Results and Discussion
Characterization of the Copolymers
The scheme of the graft copolymerization process of citronellyl methacrylate monomer onto starch backbone is shown in Fig. 3. The grafting parameters obtained for the different starch to citronellyl methacrylate ratio are presented in Table 1.
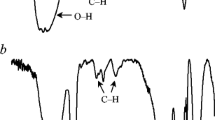
The presence of the stretching vibrations for –OH groups above 3500 cm−1 on the ATR-FTIR spectrum and the counted %G confirmed only partial substitution of hydroxyl groups of starch by poly(citronellyl methacrylate) chains. According to the calculated grafting percent (%G) from 0.3 to ca. 1.6 hydroxyl groups per glycoside unit were substituted by new groups. Figure 4 showed the FTIR spectrum for the examplary copolymer. It was well visible the presence of the stretching vibrations of C–H at maxima centered from 2865 to 2950 cm−1, the stretching vibration of C=O at 1726 cm−1, two signals with maxima at 1370–1375 and at 1445–1460 cm−1 which were characteristic for the deformation vibrations of C–H, the high intensity signals from 1000 to 1300 cm−1 responsible for the stretching vibrations of C–O and the vibrations below 930 cm−1 which led to the presence of the skeletar vibrations of C–H and the out-of-plane deformation vibrations of =C–H. The solid state NMR spectra also affirmed the structure of the copolymers. Besides the signals responsible for the carbon atoms present in the structure of starch from 6 to 102 ppm (C1–C6), the appearance of the additional signals connecting with the presence of carbon atoms in C=O groups at 175–177 ppm and the carbon atoms in CH, CH2 and CH3 groups from 20 to 45 ppm confirmed the formation of the desired product, (Fig. 4).
The SEM imagines showed that the morphology of the obtained materials differed considerably as compared to raw potato starch [21]. The incorporation of poly(citronellyl methacrylate) chains onto starch resulted in the destruction of starch grains and creation of irregular, connected, flap forms with concise but containing some cracks surface. Moreover, based on the SEM imagines it was noticed that as the grafting percent was increased the flaps were higher and more evident, (Fig. 5).
Physicochemical Properties of Copolymers
The swellability coefficient values for the obtained copolymers and raw potato starch in different solvents (polar: water, ethanol, butanol and non-polar: hexane, toluene and CCl4) were shown in Fig. 6a. The chemical modification of polar potato starch changed drastically the polarity of the copolymers which resulted in its lower swelling in polar solvents and its higher swelling in non-polar solvents as compared to unmodified potato starch [21, 22].
The chemical resistance of the obtained starch-g-poly(citronellyl methacrylate) copolymers in 1 M HCl, 1 M NaOH and buffer solutions with the following pH 5, 7 and 9 has been studied. The studies indicated that the grafting of poly(citronellyl methacrylate) chains onto starch backbone caused considerably changes in the chemical stability of the prepared copolymers as compared to raw potato starch. In 1 M NaOH solution, the copolymers with lower grafting percent (copolymer 1 and copolymer 2) and raw potato starch were fully degradated. However as the grafting percent was above 20%, their chemical resistance in alkaline conditions significantly increased. Moreover, as it was cleary seen, the chemical resistance of the copolymers in all solutions was increased as the grafting percent increased. What was interesting was that the highest chemical stability of copolymers in buffer with pH 7 was indicated, (Fig. 6b).
According to the results presented in Fig. 6c, the starch-g-poly(citronellyl methacrylate) copolymers were characterized by considerably lower percent moisture absorbance than raw potato starch. The percent moisture absorbance for copolymer with the highest grafting percent (copolymer 7) was ca. 5 times lower as compared to potato starch. This was predictable situation since the introduction of hydrophobic poly(citronellyl metharylate) chains into the structure of starch resulted in obtaining more hydrophobic copolymers with concise, non-porous structure with not many cracks and thus lower absorption of hydrophilic nature moisture.
Thermal Properties and Decomposition Mechanism of Copolymers
On the TG/DTG curves, one can see several temerature ranges where the mass loss under the heating of starch-g-poly(citronellyl methacrylate) copolymers in inert conditions is observed, (Fig. 7). The first one was visible at T max0 from ca. 81 °C to ca. 130 °C. It was connected with small mass loss (0.1–2.8%), (Table 2). It was due to the evaporation of moisture from the copolymers which was confirmed based on the FTIR analysis. On the gaseous FTIR spectra gathered at T max0 the presence of the signals characteristic for water vapour visible as the “noises” at 1400–1700 cm−1 and at 3500–3900 cm−1 has been indicated [33], (Fig. 8). The second mass loss between the tempeartures of 140–215 °C with T max1 at 161–194 °C for the majority of the copolymers (without copolymer 1) was appeared. In this stage, the beginning of the decomposition of copolymers was happened. The mass loss in this decomposition stage was from 2.9% up to 7.9%. Analyzing the gaseous FTIR spectra gathered at T max1, the presence of the absorption signals responsible for the stretching vibrations of C–H at 2734–2977 cm−1, the small signal at 3068 cm−1 coming from the stretching vibrations of =C–H, the undivided signals at 1700–1790 cm−1 which were the result of the stretching vibrations characteristic for C=O groups, the signal at 1643 cm−1 describing the stretching vibrations of C=C, the signals at 1508–1540 cm−1 responsible for the stretching vibrations of ring C=C, the small signals at the range of 1344–1470 cm−1 responsible for the deformation vibrations of C–H, the signals at 1066–1203 cm−1 characteristic for the stretching vibrations of C–O and the signals with low intensity below the wavenumber of 900 cm−1 which were due to the out of plane deformation vibrations of =C–H has been observed [33]. The occurrence of the above mentioned absorption signals on the FTIR spectrum indicated on long chain scissors and the emisssion of small amounths of aldehydes, alcohols, acids, aliphatics and furanes [34, 35].
The next decomposition stage of the copolymers run at the temperature range of ca. 215–320 °C with T max2 at 275–283 °C. The mass loss was from 41.9% for copolymer 1 to 29.9% for copolymer 7 and it was decreased as the %G increased. On the gaseous FTIR spectra collected at T max2, the characteristic bands for water (above 3500 cm−1), carbon oxide (2000–2200 cm−1), carbon dioxide (2300–2352 cm−1), the stretching vibrations for C–H groups (2730–2970 cm−1), the stretching vibrations for C=O (1700–1790 cm−1), the stretching vibration for C=C (1645 cm−1), the stretching vibrations for C=C at a ring (1510–1540 cm−1), the deformation vibrations for C–H (1365–1450 cm−1), the stretching vibrations for C–O (1070–1286 cm−1) and the out of plane deformation vibrations for =C–H (740–990 cm−1) [33] which indictated on the emission of the mixture of gaseous decomposition products were appeared. Among them, the evolution of aldehyde, acids, alcohols, aliphatic, alkene and furanes fragments are the most expected as a result of the decomposition of starch from the copolymers. In addition, the presence on the gaseous FTIR spectra accumulated at T max2 some of the bands indicated on the beginning of the emisssion of monomer: citronellyl methacrylate and some of alkene and acid as a result of the chain end depolymerization process of the grafted polymer and the decomposition of monomer. According to the studies performed in our Department, decomposition of pure poly(citronellyl methacrylate) was described by wide, assymetrical DTG peak which spread between the temperatures of ca. 250 °C up to ca. 500 °C with T max ca. 396 °C. It confirmed that the depolymerization initiated at the end of polymer chain could happened under the second decomposition stage of copolymers.
The third decomposition stage which was not completely separated from the second decomposition stage was happened from the temperature of ca. 320 °C to ca. 540 °C with T max3 366–393 °C. The mass loss was from 23.4% up to 50.4% and it was increased as the %G increased. It indicated that this decomposition stage was directly connected with the decomposition of the grafted poly(citronellyl methacrylate). This was confirmed based on the gathered FTIR spectra at T max3. On the FTIR spectra, the occurrence of the bands characteristic for the stretching vibrations for =C–H (3080 cm−1), the stretching vibrations for C–H (2879–2966 cm−1), the stretching vibrations for C=O (1700–1763 cm−1), the stretching vibrations for C=C (1645 cm−1), the deformation vibrations for C–H (1388 and 1460 cm−1), the stretching vibrations for C–O (1039–1290 cm−1) and the out of plane deformation vibrations for =C–H (802–910 cm−1) indicated on the emission of monomer (citronellyl methacrylate) as a main decomposition product which was the result of the depolymerization process of grafted polymer. In addition, the double carbonyl signal, wide signal at 1030–1290 cm−1 and the emission of CO2, CO and H2O in this decomposition stage may indicate on the partial decarboxylation of the main product and the emission of some lower molecular mass compounds such as acids, aldehydes and alkenes as a result of the decomposition of monomer. Finally, at the temperatures above 540 °C, slow mass loss in the range of 2.9–1.1% was observed. At this decomposition stage, the main decomposition products were water and methane. It was affirmed based on the presence of the water bands (1400–1700 cm−1 and above 3500 cm−1) and methane band (3014 cm−1) [33] on the gaseous FTIR spectra. Aditionally, it was clearly visible that the methane band intensity was decreased as the grafting percent increased. It indicated that the amounth of methane created at this decompsoition stage could be connected and dependent on the presence of potato starch in the copolymers. According to the results presented in Table 2, the heating of the copolymers up to 850 °C caused the formation of high char content (29.3–13.5%). It proved that the starch-g-poly(citronellyl methacrylate) copolymers in particular those with lower grafting percent can be considered as potential precursors for the preparation of carbon materials [36, 37].
Conclusions
The chemical modification of potato starch with citronellyl methacrylate monomer under the grafting process allowed obtaining novel, more environmentally friendly with improved physicochemical properties and cheaper amphiphilic materials.
It was proved that the incorporation of poly(citronellyl methacrylate) chains onto starch resulted in the destruction of starch granules and creation of irregular, concise with some cracks surface. The chemical modification of potato starch with the hydrophobic polymer allowed producing the materials which were characterized by higher chemical stability towards acid, alkaline and buffers environment, higher moisture resistance, higher swelling in non-polar solvents, higher solvent resistance as compared to unmodified potato starch. Additionally, thermal decomposition process of copolymers happened at least four stages connecting with the evolution of various decomposition products in each stage. Due to their properties the novel materials can be applied as stabilizers, modifiers, fillers, matrices, plastics, excipiens for the specific drug delivery systems or as potential precursors for the preparation of carbon materials.
References
Ait Ali M, Allaoud S, Karim A, Roucoux A, Mortreux A (1995) Tetrahedron 6:369–370
Wheeler JW, Ibrahim SA, Weldon PJ (1999) Biochem Syst Ecol 27:27–32
Taylor WG, Schreck CE (1985) J Pharm Sci 74:534–539
Morris RH (2007) In: de Vries JG and Elsevier CJ (eds) The handbook of homogeneous hydrogenation. Wiley, Weinheim
Songkro S, Hayook N, Jaisawang J, Maneenuan D, Chuchome T, Kaewnopparat N (2012) J Incl Phenom Macrocycl Chem 72:339–355
Burdock GA (2005) Fenaroli’s handbook of flavor ingredients. CRC Press, Cleveland
Alsters PL, Jary W, Nardello-Rataj V, Aubry JM (2010) Org Process Res Dev 14:259–262
Bauer K, Garbe D, Surburg H (2001) Common fragrance and flavor materials: preparation, properties and uses. Wiley, New York
Habulin M, Sabeder S, Paljevac M, Primozˇicˇ M, Knez Z (2007) J Supercrit Fluids 43:199–203
Croteau R (1980) Fragrance and flavor substances, D&PS Verlag, Pattensen
United State Patent (1977) 5652205 Perfumes for laundry and cleaning compositions
United State Patent (1996) PTC/US1995/008965 Manufacture of perfumes for laundry and cleaning
Gildemeister E, Hoffmann FR (1913) The volatile oils. Wiley, New York
Melo LLMM, Pastore GM, Macedo GA (2005) Process Biochem 40:3181–3185
Chatterjee BKDT, Bhattacharyya DK (1999) J Am Oil Chem Soc 76:1501–1504
Worzakowska M (2015) Sposób otrzymywania tetraestrów zapachowych. Patent RP 221455.
Worzakowska M (2014) J Therm Anal Calorim 118:299–309
Worzakowska M (2015) J Therm Anal Calorim 121:235–243
Athavale V, Manjrekar N, Athawale M (2003) Biotechnol Prog 19:298–302
Grochowicz M, Gawdzik B (2013) J Porous Mater 20:339–349
Worzakowska M, Grochowicz M (2015) Carbohydr Polym 130:344–352
Worzakowska M (2016) J Therm Anal Calorim 124:1309–1318
Worzakowska M (2017) J Therm Anal Calorim. doi:10.1007/s10973-017-6182-y
Lim ST, Lee JH, Shin DH, Lim HS (1999) Starch/StÓ“rke 51:410–415
Sokrates G (2001) Infrared and Raman characteristic group frequencies, tables and charts. Wiley, New York
Athawale VD, Rathi SC (1997) React Funct Polym 34:11–17
Fares MM, El-faqeeh AAS, Osman ME (2003) J Polym Res 10:119–125
Fakhru’l-Razi A, Qudsieh IYM, Yunus WMZW, Ahmad MB, Rahman MZA (2001) J Appl Polym Sci 82:1375–1381
Tuncel K, Ecevit K, Kesenci K, Piskin E (1996) J Polym Sci Part A 34:45–55
Pathania D, Sharma R (2012) Adv Mater Lett 3:136–142
Kaith BS, Singha AS, Grupa SK (2003) J Polym Mater 20:195–199
NIST Chemistry Webbook, NIST standard reference data 2011, http://webbook.nist.gov
Liu X, Wang Y, Yu L, Tong Z, Chen L, Liu H, Li X (2013) Starch-Starke 65:48–60
Liu X, Yu L, Xie F, Li M, Chen L, Li X (2010) Starch-Starke 62:139–146
Yu J, Gao LZ, Li XL, Wu C, Gao LL, CM Li (2016) New Carbon Mater 31:475–484
Zhao M, Li B, Cai JX, Liu C, McAdam KG, Zhang K (2016) Fuel Process Technol 153:43–49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Worzakowska, M. Chemical Modification of Potato Starch by Graft Copolymerization with Citronellyl Methacrylate. J Polym Environ 26, 1613–1624 (2018). https://doi.org/10.1007/s10924-017-1062-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1062-x