Abstract
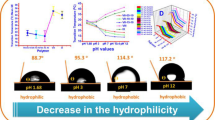
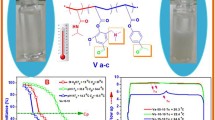
The study aims to synthetize a new functional acrylate monomer from vanillin in one step reaction. The new monomer has called by vanillin acrylate and abbreviated (VA). It was confirmed by chemical methods 1H NMR, 13C, IR and UV indicated logic results. The homopolymer has been prepared by free radical polymerization and characterized chemically and physically, the 1HNMR and IR have proofed the presence of aldehyde group in the polymer main chain. This monomer was used to synthesize three different molar concentrations of temperature responsive functional polymers with N-isopropylacrylamide via free radical polymerization by AIBN in solution. The 1H NMR was used for determination of actual mole concentration of each monomer. The aldehyde group in polymers were used as a linker for grafting with primary amine compounds by click reactions to form Schiff’s base imine compounds. The grafted copolymers were also investigated by 1H NMR and IR for structure elucidation. Gel permeation chromatography GPC was used for determination the molecular weight and polydispersity; DSC for glass temperature of solid polymers; XRD for crystallinity. UV–Vis Spectroscopy was used for the determination of phase separation or the lower critical solution temperature (Tc) of polymers solution before and after grafting, not only in deionized water but in pH 5 and pH 11.
Graphical Abstract
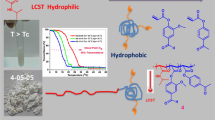
Synthesis of new functional monomer based on vanillin in one step reaction. Monomer has been successfully polymerized by free radical polymerization using AIBN as initiator. Three different mole ratios (10, 15, 20 mol%) of copolymer with NIPAAm were prepared. All monomers and polymers have been chemically elucidated. The aldehyde functional group used as linker for primary amine compounds, the final products have been characteristics as T, pH dual responsive polymers the phase transition temperature of copolymer before and after grafting was determined by UV.vis Spectroscopy.













Similar content being viewed by others
References
Abdelaty MSA, Kuckling D (2016) Gels 2:13
Deshmukh PK, Ramani KP, Singh SS, Tekade AR, Chatap VK, Patil GB, Bari SB (2013) J Control Release 166:306
Kikuchi A, Okano T (2002) Prog Polym Sci, 27:1193
Ware T, Simon D, Rennaker RL, Voit W (2013) Smart polymers for neural interfaces. Polym Rev 53:129
Hamner KL, Alexander CM, Coopersmith K, Reishofer D, Provenza C, Maye MM (2013) ACS Nano 7:7020
Qiu Y, Park K (2001) Adv Drug Deliv Rev 53:339
Sato E, Masuda Y, Kadota J, Nishiyama T, Horibe H (2015) Eur Polym J 69:615
Chen J-K, Chang C-J (2014) Materials 7:875
Meng H, Mohamadian H, Stubblefield M, Jerro D, Ibekwe S, Pang SS, Li GQ (2013) Smart Mater Struct 22:9
Zhang M, Estournes C, Bietsch W, A.H.E. Mueller (2004) Adv Funct Mater, 14:882
Matsukuma D, Yamamoto K, Aoyagi T (2006) Langmuir 22:5915
Chen Y, Pang X-H, Dong C-M (2010) Adv Funct Mater 20:586
Schattling P, Jochum F-D, Theato P (2014) Poly Chem, 5:36
Li Y, Zhang C, Zhou Y, Dong Y, Chen W (2015) Eur Polym J 69:448
Fujiwara N, Asaka K, Nishimura Y, Oguro K, Torikai E (2000) Chem Mater, 12:1754
Roy D, Cambre JN, Sumerlin B (2011) in Handbook of Stimuli-Responsive Materials (Ed.: M. W. Urban). Wiley-VCH, Weinheim
Xia Y, Yin X, Burke NAD, Stoever HDH (2005) Macromolecules 38:5943
Cheng G, Boeker A, Zhang M, Krausch G, Mueller AHE (2001) Macromolecules 34:6888
Chen JK, Wang JH, Fan SK, Chang JY (2012) J Phys Chem C 116:6992
Seuring J, Agarwal S (2012) Macromol Rapid Commun, 33:1920
Chang K, Rubright NC, Lowery PD, Taite LJ (2013) J Polym Sci APolym Chem, 51:2078
M. Heskins, Guillet JE (1968) J Macromol Sci Chem A 2:1455
Suwa K, Morishita K, Kishida A, Akashi M (1997) J Polym Sci A Polym Chem 35:3094
Schild HG (1992) Prog Polym Sci 17:249
Okubo M, Ahmad H, Suzuki T (1998) Colloid Polym Sci 276:475
Iatridi Z, Mattheolabakis G, Avgoustakis K, Tsitsilianis C (2011) Soft Matter 7:11169
Liu X, Yu D, Jin C, Song X, Cheng J, Zhao X, Qi X, Zhang G (2014) New J Chem 38:4830
Soppimath KS, Tan DC-W, Yang Y-Y (2005) Adv Mater 17:326
Delcea M, Möhwald H, Skirtach AG (2011) Adv Drug Deliv Rev 63:747
Uhlig K, Boysen B, Lankenau A, Jaeger M, Wischerhoff E, Lutz J-F, Laschewsky A, Duschl C (2012) Biomicrofluidics 6:11
Skirtach AG, Yashchenok AM, Möhwalda H (2011) Chem Commun 47:12746
Bedard M, Skirtach AG, Sukhorukov GB (2007) Macromol Rapid Commun 28:1521
Honda M, Kataoka K, Seki T, Takeoka Y (2009) Langmuir 25:8356
Fleischmann EK, Zentel R (2013) Angew Chem Int Ed, 52:8827
Zhang C, Madbouly SA, Kessler MR (2015) MacromolChemPhys 216:1822
Stanzione JF, Sadler JM, La Scala JJ, Wool RP (2012) ChemSusChem 5:1297
Fache M, Darroman E, Besse V, Auvergne R, Caillol S, Boutevina B (2014) Green Chem 16:1998
Ananda SA, Bernard W, Ashfaqur R (2012) Green Chem 14:2397
Ananda SA, Ashfaqur R (2012) ISRN Polym Sci 3:5
Mohammed IA, Hamidi RM (2012) Molecules 17:656
Firdausand M, Meier AR (2013) Eur Polym J, 49:166
Mialon L, Vanderhenst R, Pemba AG, Miller SA (2011) Macromol Rapid Commun, 32:1392
Srinivasa V, Rao Samui AB (2008) Polym Chem, 46:7655
Sini NK, Bijwe J, Varma IK (2014) J Polym Sci Part A 52:11
Shimasaki T, Yoshihara S, Shibata M (2012) Polym Compos 33:1847
Xin Y, Yuan J (2012) Polym Chem 3:3055
Zhou L, Cai Z, Yuan J, Kang Y, Yuan W, Shen D (2011) Polym Int 60:1308,
Etika KC, Cox MA, Grunlan JC (2010) Polymer 51:1770
Y. Oda, S. Kanaoka, S. Aoshima (2010) J. Polym Sci Part A 48:1213
Yan Q, Zhou R, Fu C, Zhang H, Yin Y, Yuan J (2011) Angew Chem Int Ed 50:4930
Dondoniand A, Marra A (2012) Chem Soc Rev 41:586
Fu R, Fu G (2011) Polym Chem 2:475
Francand G, Kakkar AK (2010) Chem Soc Rev 39:1544
Iha RK, Wooley KL, Nyström AM, Burke DJ, Kadeand MJ, Hawker CJ (2009) Chem Rev 109:5686
Gupta S, Kuckling D, Kretschmer K, Choudhary V, Adler H-J (2007) J Polym Sci 45:679
Acknowledgements
The authors are grateful acknowledge to Egyptian culture and missions, and The Deutscher Akademischer Austauch (DAAD) for financial assistance during the post doctor work in Germany of Momen S.A. Abdelaty.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A. Preparation and Characterization of New Environmental Functional Polymers Based on Vanillin and N-isopropylacrylamide for Post Polymerization. J Polym Environ 26, 636–646 (2018). https://doi.org/10.1007/s10924-017-0960-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0960-2