Abstract
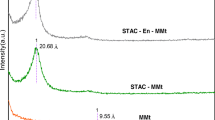
The selective modification of sodium montmorillonite (Na+-Mt) surface with polyionene followed by poly (succinimde-co-aspartate) has been considered. Na+-Mt was allowed to react with well characterized polyionene in two fold excess. The resulting polyionene/Mt (IC) was further modified with poly (succinimide-co-aspartate) through an ion exchange process. The obtained polyaspartate/Mt (IPS) composite was characterized by elemental analysis, X-ray diffraction, FTIR spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and BET surface analyzer. The adsorption efficiency of IPS composite was investigated for the removal of Pb(II) and Cd(II) from aqueous solution under different experimental conditions including initial metal ions concentration, temperature and single and binary mixture systems of metal ions. The experimental data were analyzed by Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich models. Langmuir model reveals that the monolayer adsorption capacity of IPS was 92.59 and 67.57 mg/g for Pb(II) and Cd(II), respectively. The modification of parent Na+-Mt enhanced their adsorption capacity by about 87.91 and 29.84% for Pb(II) and Cd(II), respectively, due to inclusion of extra active sites of polyaspartate. The mean sorption energy, E calculated from Dubinin–Radushkevich isotherm were 2.75 and 1.98 kJ/mol for the adsorption of Pb(II) and Cd(II), respectively, indicating physical adsorption process. Also, The thermodynamic parameters were calculated and indicated that the adsorption was spontaneous and exothermic process. The mechanism of cation exchange and complexation of metal ions was suggested. IPS composite has a considerable potential for the removal of heavy metal ions from aqueous solution and wastewater stream.











Similar content being viewed by others
References
Alateyah AI, Dhakal HN, Zhang ZY (2013) Adv Polym Technol 32:21368
Abollino O, Aceto MM, Malandrino M, Sarzanini C, Mentasti E (2003) Water Res 37:1619
Hemond HF, Fechner EJ (1994).) Chemical fate and transport in the environment. (Academic Press, San Diego
Bergaya F, Lagaly G (2013) In: Handbook of Clay Science, (eds.) Bergaya F, Lagaly G (Elsevier, Amsterdam pp 2–15
Zhu R, Chen Q, Zhu R, Xu Y, Ge F, Zhu J, He H (2015) Appl Clay Sci 107:90
Xi Y, Frost RL, He H, Kloprogge T, Bostrom T (2005) Langmuir 21:8675
Osman MA, Ploetze M, Skrabal P (2004) J Phys Chem B 108:2580
Heinz H, Vaia RA, Krishnamoorti R, Farmer BL (2007) Chem Mater 19:59
Sharlene RW, Timothy EL (2009) Prog Polym Sci 34:762
Rembaum A, Baumgartner W, Eisenberg A (1968) J Polym Sci Polym Lett Ed 6:159
Gibbs CF, Littman ER, Marvel CS (1933) J Am Chem Soc 55:753
Erdmenger T, Perevyazko I, Vitz J, Pavlov G, Schubert US (2010) J Mater Chem 20:3583
Eisenberg A, King M (1977) Ion-containing polymers: physical properties and structure. (Academic Press, New York)
Trukhanova ES, Litmanovich AA, Zelikin AN (2005) Biomacromolecules 6:3198
Li F, Cheng F, Shi J, Cai F, Liang M, Chen J (2007) J Power Sources 165:911
He X, Chan TH (2007) Org Lett 9:2681
Liu Y, Li J, Yang Y, Li B (2015) Appl Surf Sci 351:831
Fu FL, Wang Q (2011) J Environ Manag 92:407
Hua M, Zhang SJ, Pan BC, Zhang WM, Lv L, Zhang QX (2012) J Hazard Mater 211:317
Mascia M, Vacca A, Palmas S (2015) J Chem Technol Biotechnol 90:1290
Wang J, Chen C (2009) Biotechnol Adv 27:195
Kılıç M, Kırbıyık Ç, ˘ullar Çepeliog Ö, Pütün AE (2013) Appl Surf Sci 283:856
Gupta VK, Carrott P, Carrott MR (2009) Environ Sci Technol 39:783
Ge F, Li MM, Ye H, Zhao BX (2012) J Hazard Mater 211–212:366
Gao J, Sun S, Zhu W, Chung T (2014) Water Res 63:252
Lee SM, Tiwari D (2012) Appl Clay Sci 59–60:84
Wang CP, Wu JZ, Sun HW, Wang T, Liu HB, Chang Y (2011) Ind Eng Chem Res 50:8515
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huang Y, Wang X (2011) Dalton Trans 40:1095
Chen Y, Zhang W, Yang S, Hobiny A, Alsaedi A, Wang X (2016) Sci China Chem 59:412
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Environ Sci Technol 45:10454
Yang G, Jiang H (2014) Water Res 48:396
Moreno-Barbosa J, López-Velandia C, Maldonado A, Giraldo L, Moreno-Piraján J (2013) Adsorption 19:675
Kraepiel AML, Keller K, Morel FMM (1999) J Colloid Interface Sci 210:43
Bradbury MH, Bayens B (1999) Geochim Cosmochim Acta 63:325
Bayens B, Bradbury MH (1997) J Contam Hydrol 27:199
Ikhsan J, Wells JD, Johnson BB, Angove MJ (2005) Colloids Surf A 252:33
Yariv S, Cross H (2002) Organo-Clay Complexes and Interactions. (Marcel Dekker, New York)
Constantinos GT, Christidis SG, Favvas EP (2013) Fuel 104:155
Jaynes WF, Boyd SA (1991) Soil Sci Soc Am J 55:43
Kozaka M, Domkab L (2004) J Phys Chem Solids 65:441
Sakizci M, Alver BE, Alver O, Yorukogullari E (2010) J Mol Struct 969:187
Lakshmi MS, Sriranjani M, Bakrudeen HB, Kannan AS, Mandal AB, Reddy BSR (2010) Appl Clay Sci 48:589
Claudia RE, Mansur RS, Oliveira VA, Yure GC, Queiro ´s, Luciana SS, Elizabete FL (2012) J Appl Polym Sci 123:218
Hilary I, Inyanga AO, Bae S (2016) Soil Tillage Res 155:124
Langmuir I (1918) J Am Chem Soc 40:1361
Freundlich HMF (1906) Z Phys Chem 57:385
Huang Y, Yang C, Sun Z, Zeng G, He H (2015) RSC Adv 5:11475
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R (2013) Dalton Trans 42:5682
Unuabonah EI, Olu-Owolabi BI, Adebowale KO, Yang LZ (2008) Adsorp Sci Technol 26:383
Weber TW Jr, Chakravorty RK (1974) AIChE J 20:228
Naiya TK, Bhattacharya AK, Das SK (2009) J Colloid Interface Sci 333:14
Do DD (1998) Adsorption analysis: equilibria and kinetics (Imperial College Press, London)
Dubinin MM, Radushkevich LV (1947) Proc Acad Sci USSR Phys Chem Sect 55:331
Chen AH, Liu SC, Chen CY, Chen CY (2008) J Hazard Mater 154:184
Chen AH, Yang CY, Chen CY, Chen CW (2009) J Hazard Mater 163:1068
Rasouli M, Yaghobi N, Hafezi M, Rasouli M (2012) J Ind Eng Chem 18:1970
Katal R, Vafaie Sefti M, Jafari M, Saeedi DA, Sharifian M, Ghayyem MA (2012) J Ind Eng Chem 18:230
Smith JM, Van Ness HC, Abbott MM (2004) Introduction to chemical engineering thermodynamics. (McGraw-Hill, New York)
Sharma P, Das MR (2013) J Chem Eng Data 58:151
Kılıc M, Kırbıyık C, Cepeliogullar Ö, Pütün AE (2013) App Surf Sci 283:856
Karthik R, Meenakshi S (2015) Desalin Water Treat 56 1587
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH)—King Abdulaziz City for Science and Technology—the Kingdom of Saudi Arabia—award number (11-NAN2029-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support” The authors also acknowledge Dr. Mohamed El-Newehy and Dr. Hany El-Hamshary for providing helping in discussion the characterization of the composite.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Elsherbiny, A.S., El-Hefnawy, M.E. & Gemeay, A.H. Adsorption Efficiency of Polyaspartate-Montmorillonite Composite Towards the Removal of Pb(II) and Cd(II) from Aqueous Solution. J Polym Environ 26, 411–422 (2018). https://doi.org/10.1007/s10924-017-0958-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0958-9