Abstract
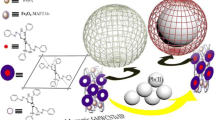
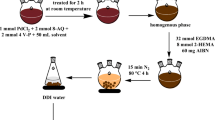
In this study, a novel magnetic Cr(VI) ion imprinted polymer (Cr(VI)-MIIP) was successfully synthesized and used as a selective sorbent for the adsorption of Cr(VI) ions from aqueous solution. It can be synthesized through the combination of an imprinting polymer and magnetic nanoparticles. The high selectivity achieved using MIIP is due to the specific recognition cavities for Cr(VI) ions created in Cr(VI)-MIIP. Also, the magnetic properties that could be obtained using magnetic nanoparticles, helps to separate adsorbent with an external magnetic field without either additional centrifugation or filtration procedures. The magnetic Fe3O4 nanoparticles (MNPs) were synthesized using an improved co-precipitation method and modified with tetraethylorthosilicate (TEOS) before imprinting. The magnetic Cr(VI) ion imprinted polymer was prepared through precipitation copolymerization of 4-vinylpyridine as the complexing monomer, 2-hydroxyethyl methacrylate as a co-monomer, the Cr6+ anion as a template, and ethylene glycol dimethacrylate (EGDMA) as a cross-linker in the presence of modified magnetite nanoparticles. This novel synthesized sorbent was characterized using different techniques. Batch adsorption experiments were performed to evaluate the adsorption conditions, selectivity, and reusability. The results showed that the maximum adsorption capacity was 39.3 mg g−1, which was observed at pH 3 and at 25 °C. The equilibrium time was 20 min, and the amount of adsorbent which gave the maximum adsorption capacity was 1.7 g L−1. Isotherm studies showed that the adsorption equilibrium data were fitted well with the Langmuir adsorption isotherm model and the theoretical maximum adsorption capacity was 44.86 mg g−1. The selectivity studies indicated that the synthesized sorbent had a high single selectivity sorption for the Cr(VI) ions in the presence of competing ions. Thermodynamic studies revealed that the adsorption process was exothermic (\(\Delta H\) < 0) and spontaneous (\(\Delta G\) < 0). In addition, the spent MIIP can be regenerated up to five cycles without a significant decrease in adsorption capacity.









Similar content being viewed by others
References
Kot A, Namiesnèik J (2000) Trends Anal Chem 19:69
Owlad M, Aroua MK, Daud WAW, Baroutian S (2009) Water Air Soil Pollut 200:59
Testa JJ, Grela MA, Litter MI (2004) Environ Sci Technol 38:1589
Xing YQ, Chen XM, Wang DH (2007) Environ Sci Technol 41:1439
Mohan D, Pittman Jr. CU (2006) J Hazard Mater B 137:762
Dubey SP, Gopal K (2007) J Hazard Mater 145:465
Kozlowski CA, Walkowiak W (2002) Water Res 36:4870
Sun JM, Li F, Huang JC (2006) Ind Eng Chem Res 45:1557
Clevenger T, Novak JT (1983) J Water Pollut Control Fed 55:984
Fraser BG, Pritzker MD (1994) Sep Sci Technol 29:2097
Ouk SK, Neufeld RD (1997) J Chem Technol Biotechnol 70:3
Mohanty K, Jha M, Meikap BC, Biswas MN (2006) Chem Eng J 117:71
Yang J, Yu M, Qiu T (2014) J Ind Eng Chem 20:480
Lu Y, Yan CL, Gao SY (2009) Appl Surf Sci 255:6061
Araki K, Maruyama T, Kamiya N, Goto M (2005) J Chromatogr B 818:141
Singh DK, Mishra S (2009) J Hazard Mater 164:1547
Hoai NT, Yoo DK, Kim D (2010) J Hazard Mater 173:462
Srividya K, Mohanty K (2009) Chem Eng J 155:666
Arica MY, Bayramoglu G (2005) Colloids Surf A 253:203
Chen L, Wang X, Lu W, Wu X, Li J (2016) Chem Soc Rev 45:2137
Özcan AA, Demirli Ş (2014) Sep Sci Technol 49:74
Chen L, Xu S, Li J (2011) Chem Soc Rev 40:2922
Ahmadi SJ, Noori KO, Shirvani AS (2010) J Hazard Mater 175:193
Andac M, Özyapı E, Senel S, Say R, Denizli A (2006) Ind Eng Chem Res 45:1780
Cai X, Li J, Zhang Z, Yang F, Dong R, Chen L (2014) ACS Appl Mater Interfaces 6:305
Xu S, Chen L, Li L, Guan Y, Lu H (2012) J Hazard Mater 237–238:347
Fu J, Chen L, Li J, Zhang Z (2015) J Mater Chem A 3:13598
Lee SC, Patil UM, Kim SJ, Ahn S, Kang SW, Jun SC (2016) RSC Adv 6:44087
Birlik E, Buyuktiryaki S, Ersoz A, Say R, Denizli A (2006) Sep Sci Technol 41:3109
Gao BJ, Wang J, An FQ, Liu Q (2008) Polymer 49:1230
Ebrahimzadeh H, Moazzen E, Amini MM, Sadeghi O (2013) Chem Eng J 315:215
Liu Y, Liu Z, Gao J, Dai J, Han J, Wang Y, Xie J, Yan Y (2011) J Hazard Mater 186:197
An FQ, Gao BJ, Feng XQ (2008) J Hazard Mater 157:286
Ren YM, Wei XZ, Zhang ML (2008) J Hazard Mater 158:14
Zhao YG, Shen H, Pan SH, Hu M (2010) J Hazard Mater 182:295
Chen L, Liu J, Zeng Q, Wang H, Yu A, Zhang H, Ding L (2009) J Chromatogr A 1216:3710
Zhang ML, Zhang ZH, Liu YN, Yang X, Luo LJ, Chen JT, Yao SZ (2011) Chem Eng J 178:443
Li J, Dong R, Wang X, Xiong H, Xu S, Shen D, Song X, Chen L (2015) RSC Adv 5:10611
Pakade V, Cukrowska E, Darkwa J, Torto N, Chimuka L (2011) Water SA 37:529
Bayramoglu G, Arica MY (2011) J Hazard Mater 187:213
Marjanović V, Lazarević S, Janković-Častvan I, Potkonjak B, Janaćković Ð, Petrović R (2011) Chem Eng J 166:198
Duranoğlu D, Kaya IGB, Beker U, Filiz SB (2012) Chem Eng J 181–182:103
Tavengwa NT, Cukrowska E, Chimuka L (2013) Talanta 116:670
Faraji M, Yamini Y, Tahmasbi E, Saleh A, Nourmohammadian F (2010) J Iran Chem Soc 7:130
Prasanna Kumar Y, King P, Prasad VSRK (2007) Chem Eng J 129:161
Azizian S (2004) J Colloid Interface Sci 276:47
Ho YS, McKay G (1998) Chem Eng J 70:115
Langmuir I (1918) J Am Chem Soc 40:1361
Freundlich HMF (1906) Z Phys Chem 57:385
Webi TW, Chakravort RK (1974) AIChE J 20:228
Wang XS, Chen LF, Li FY, Chen KL, Wan WY (2010) J Hazard Mater 175:816
Xing GX, Zhang SF, Ju BZ, Yang JZ (2006) Carbohydr Polym 66:246
Cheng RM, Ou SJ, Xiang B, Li YJ, Liao QQ (2009) J Polym Res 16:703
Bayramoglu G, Arica MY (2005) Sep Purif Technol 45:192
Huang GL, Shi JX, Langrish TAG (2009) Chem Eng J 152:434
Liu W, Zhang J, Zhang C, Wang Y, Li Y (2010) Chem Eng J 162:677
Janos P, Hula V, Bradnova P, Pilarova V, Sedlbauer J (2009) Chemosphere 75:732
Hu J, Chen GH, Lo IMC (2005) Water Res 39:4528
Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L (2008) Bioresour Technol 99:6271
Lv X, Xu J, Jiang G, Tang J, Xu X (2012) J Colloid Interface Sci 369:460
Tan T, He X, Du W (2001) J Chem Technol Biotechnol 76:191
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) J Hazard Mater 184:126
Acknowledgements
This work was financially supported by the Iranian Nanotechnology Initiative Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassanpour, S., Taghizadeh, M. & Yamini, Y. Magnetic Cr(VI) Ion Imprinted Polymer for the Fast Selective Adsorption of Cr(VI) from Aqueous Solution. J Polym Environ 26, 101–115 (2018). https://doi.org/10.1007/s10924-016-0929-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0929-6