Abstract
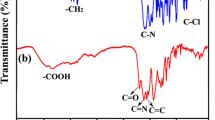
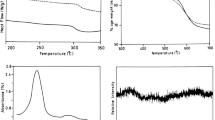
A short and green method for the preparation of optically active aromatic polyamides (PAs) using tetrabutylammonium bromide (TBAB) as a molten ionic liquid is reported. Polycondensation reactions of amino acid containing diacid (2S)-5-(4-methyl-2-phthalimidylpentanoylamino)isophthalic acid with various commercially available diisocyanates in molten TBAB as a green medium or in N-methylpyrrolidone as common organic solvent with or without dibutyltin dilaurate as a catalyst under microwave irradiation were carried out. Various PAs were obtained with high yields and moderate inherent viscosities in the rang of 0.30–0.57 dL/g. The obtained polymers were characterized by FT-IR, specific rotation measurements, and representative of them by 1H NMR and elemental analysis techniques. Thermal properties of PAs were evaluated by thermogravimetric analysis and the results showed that the 10% weight loss temperature in a nitrogen atmosphere for four representative samples were more than 258 °C, which indicates that the resulting PAs have good thermal stability as well as excellent solubility.




Similar content being viewed by others
References
Chai WL, Chow JD, Chen CC, Chuang FS, Lu WC (2009) Evaluation of the biodegradability of polyvinyl alcohol/starch blends: a methodological comparison of environmentally friendly materials. J Polym Environ (in press)
Pandey JK, Raghunatha Reddy K, Pratheep Kumar A (2005) An overview on the degradability of polymer nanocomposites. Polym Degrad Stabil 88:234–250
Briassoulis D (2004) An overview on the mechanical behavior of biodegradable agricultural films. J Environ Polym Degrad 12:65–81
Joshi SS, Mebel AM (2007) Computational modeling of biodegradable blends of starch amylase and polypropylene. Carbonate Polymer 48:3893–3901
Jayasekara R, Harding I, Bowater I et al (2003) Biodegradation by composting of surface modified starch and PVA blended films. J Environ Polym Degrad 11:49–56
Xu YX, Kim KM, Hanna MA, Nag D (2005) Chitosan-starch composite film: preparation and characterization. Ind Crops Prod 21:185–192
Avella M, DeVlieger JJ, Emanuela Errico M et al (2005) Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem 93:467–474
Ren R, Shen T, Wang F, Wang X, Zhang Z (2009) Study on biodegradable starch/OMMT nanocomposites for packaging applications. J Polym Environ (in press)
Ray D, Roy P, Sengupta S, Sengupta SP, Mohanty AK, Misra M (2009) A study of physicomechanical and morphological properties of starch/poly(vinylalcohol) based films. J Polym Environ 17:56–63
Keles E, Hazer B (2009) Synthesis of segmented polyurethane based on polymeric soybean oil polyol and poly (Ethylene Glycol). J Polym Environ (in press)
Amass W, Amass A, Tighe B (1998) A Review of Biodegradable Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym Int 47:89–144
Ikada Y, Tsuji H (2000) Biodegradable polyesters for medical and ecological applications. Macromol Rapid Commun 21:117–132
Painter TJ (1998) Carbohydrate polymers in food preservation: an integrated view of the maillard reaction with special reference to discoveries of preserved foods in sphagnum-dominated peat bogs. Carbohydr Polym 36:335–347
Audic JL, Fourcade F, Chaufer B (2007) Thermodynamics, solubility and environmental issues. In: Letcher TM (ed) Biodegradable material obtained from renewable resource, Chap. 20, pp 369–382
Wathier M, Johnson CS, Kim T, Grinstaff MW (2006) Hydrogels formed by multiple peptide ligation reactions to fasten corneal transplants. Bioconjugate Chem 17:873–876
Gigante A, Chillemi C, Bevilacqua C, Greco F, Bisaccia F, Tamburro AM (2003) Effect of elastine derived peptide on achilles tendom healing. J Mater Sci Mater Med 14:717–720
Pilkington-Miksa MA, Writer MJ, Sarkar S, Meng QH, Barker SE, Shamlou PA, Hailes HC, Hart SL, Tabor AB (2007) Targeting lipopolyplexes using bifunctional peptides incorporating hydrophobic spacer amino acids: synthesis, transfection, and biophysical studies. Bioconjugate Chem 18:1800–1810
Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, Forni F (2007) Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with loperamide and rhodamine-123. J Control Rel 122:1–9
Brown MD, Gray AI, Tetley L, Santovena A, Rene J, Schatzlein AG, Uchegbu IF (2003) In vitro and invivo gene transfer with poly(amino acid) vesicles. J Control Rel 93:193–211
Billot JP, Douy A, Gallot B (1977) Preparation, fractionation, and structure of block copolymers polystyrene-poly(carbobenzoxy-L-lysine) and polybutadiene-poly(carbobenzoxy-L-lysine). Makromol Chem 178:1641–1650
Aiba S, Minoura N, Fujiwara Y, Yamada S, Nakagawa T (1985) Laminates composed of polypeptides and elastomers as a burn wound covering. Physicochemical properties. Biomaterials 6:290–296
Kopecek J (1984) Controlled biodegradability of polymers—a key to drug delivery systems. Biomaterials 5:19–25
Spatola AF (1983) Peptide backbone modifications. In: Weinstein B (ed) Chemistry and biochemistry of amino acids, peptides and proteins. Marcel Dekker, New York, pp 268–357
Kumaki T, Sisido M, Imanishi Y (1985) Antithrombogenicity and oxygen permeability of block and graft copolymers of polydimethylsiloxane and poly(α-amino acid). J Biomed Mater Res 19:785–811
Metselaar GA, Hans PJHM, Nolte RJM, Cornelissen JJLM, Rowan AE (2007) Polyisocyanides derived from tripeptides of alanine. Chem Eur J 13:950–960
Kros A, Jesse W, Metselaar GA, Cornelissen JJLM (2005) Synthesis and self-assembly of rod-rod hybrid poly(g-benzyl l-glutamate)-block-polyisocyanide copolymers. Angew Chem Int Ed 44:4349–4352
Hill DJ, Mio MJ, Prince RB, Hughes TS, Moore JS (2001) A field guide to foldamers. Chem Rev 101:3893–4011
Gemmell V (1990) VOC reduction. Solvent cleaning and paint stripping. Soc Auto Eng Trans 99:64–76
Benton MG, Brazel CS (2004) An investigation into the degree and rate of polymerization of poly(methyl methacrylate) in the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. Polym Int 53:1113–1117
Beuermann S, Buback M, Isemer C, Lacik I, Wahl A (2002) Pressure and temperature dependence of the propagation rate coefficient of free-radical styrene polymerization in supercritical carbon dioxide. Macromolecules 35:3866–3869
DeSimone JM, Maury EE, Menceloglu YZ, McClain JB, Romack TJ, Combes JR (1994) Dispersion polymerizations in supercritical carbon dioxide. Science 265:356–359
Visser AE, Swatloski RP, Reichert WM, Mayton R, Sheff S, Wierzbicki A, Davis JH, Rogers RD (2001) Task-specific ionic liquids for the extraction of metal ions from equeous solutions. Chem Commun 101:135–136
Visser AE, Swatloski RP, Reichert WM, Griffin ST, Rogers RD (2000) Traditional extractants in nontraditional solvents: groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem 39:3596–3604
Visser AE, Swatloski RP, Rogers RD (2001) So you think your process is green, how do you know? Using principles of sustainability to determine what is green-a corporate perspective. Green Chem 3:1–6
Carmichael AJ, Earle MJ, Holbrey JD, McCormac PB, Seddon KR (1999) The heck reaction in ionic liquids: a multiphasic catalyst system. Org Lett 1:997–1000
Earle MJ, McCormac PB, Seddon KR (1999) Diels-alder reactions in ionic liquids. Green Chem 1:23–25
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis WILEY-VCH Verlags GmbH & Co. KGaA, Weinheim
Keskin S, Kayrak-Talay D, Akman U, Hortacsu O (2007) A review of ionic liquids towards supercritical fluid applications. J Supercritical Fluids 43:150–180
Dupont J, de Souza RF, Suarez PA Z (2002) Ionic liquid (Molten Salt) phase organometallic catalysis. Chem Rev 102:3667–3692
Ding J, Armstrong DW (2005) Chiral ionic liquids: synthesis and applications. Chirality 17:281–292
Mallakpour S, Rafiee Z (2008) Use of ionic liquid and microwave irradiation as a convenient, rapid and eco-friendly method for synthesis of novel optically active and thermally stable aromatic polyamides containing N-phthaloyl-L-alanine pendent group. Polym Degrad Stab 93:753–759
Mallakpour S, Kowsari E (2005) Ionic liquids as novel solvents and catalysts for the direct polycondensation of N,N′-(4,4′-Oxydiphthaloyl)-bis-L-phenylalanine diacid with various aromatic diamines. J Polym Sci Part A Polym Chem 43:6545–6553
Kubisa P (2004) Application of ionic liquids as solvents for polymerization processes. Prog Polym Sci 29:3–12
Mallakpour S, Kolahdoozan M (2008) Microwave-accelerated preparation of aromatic polyamides containing phthalimide and S-valine pendant groups in ionic liquids. Iran Polym J 17:531–539
Mallakpour S, Rafiee Z (2008) Application of microwave-assisted reactions in step-growth polymerization: a review. Iran Polym J 17:907–935
Mallakpour S, Taghavi M (2009) Direct polyamidation in green media: studies on thermal degradation of novel organosoluble and optically active flame retardant polyamides. React Funct Polym 69:206–215
Mancera M, Roffe I, Al-Kass SSJ, Rivas M, Galbis JA (2003) Synthesis and characterization of new stereoregular AABB-type polyamides from carbohydrate-based monomers having D-manno and L-ido configurations. Macromolecules 36:1089–1097
Okamura A, Hirai T, Tanihara M, Yamaoka T (2002) Synthesis and properties of novel biodegradable polyamides containing α-amino acids. Polymer 43:3549–3553
Kiely D, Chen L, Lin T (2002) Synthetic polyhydroxypolyamides from galactaric, xylaric, D-glucaric, and D-mannaric acids and alkylenediamine monomers-some comparisons. J Polym Sci Part A Polym Chem 38:594–603
Garcıa-Martın MG, Perez RR, Hernandez EB, Galbis JA (2001) Synthesis of L-arabinitol and xylitol monomers for the preparation of polyamides. Preparation of an L-arabinitol-based polyamide. Carbohydr Res 333:95–103
Obst M, Steinbuchel A (2004) Microbial degradation of poly(amino acid)s. Biomacromolecules 5:1166–1176
Mallakpour S, Dinari M (2008) Microwave step-growth polymerization of 5-(4-Methyl-2-phthalimidylpentanoylamino)isophthalic acid with different diisocyanates. Polym Adv Technol 19:1334–1342
Mallakpour S, Dinari M (2009) Soluble new optically active polyamides derived from 5-(4-methyl-2-phthalimidylpentanoylamino)isophthalic acid and different diisocyanates under microwave irradiation in molten ionic liquid. J Appl Polym Sci 112:244–253
Van Krevelen DW, Hoftyzer PJ (1976) Properties of polymers. Elsevier, New York
Acknowledgments
We wish to express our gratitude to the Research Affairs Division Isfahan University of Technology (IUT), Isfahan, for partial financial support. Further financial support from National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Dinari, M. Environmentally Friendly Methodology for Preparation of Amino Acid Containing Polyamides. J Polym Environ 18, 705–713 (2010). https://doi.org/10.1007/s10924-010-0253-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-010-0253-5