Abstract
The Madras Treeshrew, Anathana ellioti (Waterhouse, 1850), is a small, poorly studied mammal from South Asia. It is the only treeshrew found across the southern half of the Indian subcontinent and is the westernmost member of its order (Scandentia). The phylogenetic relationship between Anathana and its putative sister genus, Tupaia, remains unresolved, and the morphological differences between these two genera have yet to be analyzed with craniometric data. Here we describe some features that distinguish Anathana from other treeshrews. However, our comparative morphometric analyses reveal that Anathana overlaps with Tupaia in morphospace; it is smaller than most species (including the only other species found on the Indian mainland, T. belangeri) but larger than T. minor, Dendrogale melanura, and Ptilocercus lowii. At the infraspecific level, there is some separation between eastern and western populations, but this requires further investigation with larger sample sizes. Unfortunately, Anathana is disproportionately scarce in museum collections relative to other scandentians, and additional biological surveys across South India will be critical for future research on the taxonomy, distribution, and conservation of this elusive taxon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treeshrews are small mammals endemic to South and Southeast Asia comprising the order Scandentia, which includes the families Tupaiidae and Ptilocercidae (Hawkins 2018a, b; Sargis 2020). Tupaiidae contains three genera: Tupaia, Dendrogale, and the monospecific Anathana. The Madras Treeshew, Anathana ellioti (Waterhouse 1850), also known as the Indian Treeshrew, has the westernmost geographic distribution of any treeshrew species, ranging across the southern half of the Indian subcontinent (Molur 2016; Hawkins 2018a). A large portion of the treeshrew fossil record has been found in South Asia (Sargis 2004; Sehgal et al. 2022), and it is possible that A. ellioti is the sole remaining representative of a group of extinct western lineages. Consequently, unraveling the relationship between Anathana and other treeshrews has important implications for our broader understanding of scandentian biogeography and diversification.
Molecular studies have suggested that Anathana is the sister taxon to the genus Tupaia, which includes 19 of the 23 recognized treeshrew species; however, this proposed relationship still lacks strong genetic support (Olson et al. 2005; Roberts et al. 2011). Some have suggested that Anathana should not be recognized as a distinct genus from Tupaia (Fiedler 1956) or that, at the very least, the two genera are quite closely related (Helgen 2005). In fact, the Madras Treeshrew was originally described as Tupaia ellioti Waterhouse 1850 before being described as a separate genus by Lyon (1913) on the basis of smaller canines, a shortened rostrum and premaxilla (but see Verma 1965), and small fenestrae in the zygoma (but see Verma 1965). Lyon (1913: p. 120) also mentioned some differences in the cheek teeth (“unusually well-developed hypocones”) as well as external soft-tissue features such as pelage (“coarser and harsher” relative to Tupaia) and toe pads (distinct rather than fused).
Lyon (1913) also delineated three species within the genus—A. ellioti from southeastern India, A. pallida from northeastern India, and A. wroughtoni from western India—entirely on the basis of pelage color, a plastic feature that has repeatedly proven unreliable for delimiting treeshrew taxa (see Juman et al. 2021b for a review of this issue). He noted that “[t]here are apparently no characters by which the skull and teeth of Anathana ellioti may be distinguished from those of other species in the genus” (Lyon 1913: p. 123). These taxa were later synonymized and are not currently recognized as subspecies (Corbet and Hill 1992; Helgen 2005).
The anatomical features that distinguish Anathana and Tupaia have never been assessed in modern statistical analyses. Moreover, very few specimens exist in museum collections (Helgen 2005). Here we evaluated the cranial distinctiveness of Anathana relative to other treeshrews. To do so, we conducted morphometric analyses on the largest existing dataset of Anathana specimens and an assembled dataset of other treeshrew species to quantitatively compare: 1) Anathana with the geographically closest T. belangeri specimens; 2) Anathana with seven other treeshrew species (T. belangeri, T. glis, T. minor, T. palawanensis, T. tana, Dendrogale melanura, and Ptilocercus lowii) for which craniometric data are available; and 3) the three taxa within Anathana formerly recognized by Lyon (1913) to test his hypothesis about their distinctiveness using modern statistical analyses, which have the potential to reveal subtle osteological differences among populations. Finally, we mapped the localities of Anathana specimens to visualize geographic sampling gaps.
Materials and methods
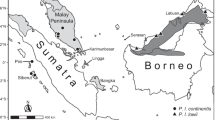
Our Anathana sample included four specimens of A. e. ellioti, six specimens of A. e. “pallida”, and two specimens of A. e. “wroughtoni” (Appendix 1; Online Resource 1). All three are represented by holotypes or paratypes in our sample (Appendix 1). Of these 12 specimens, only the eight adults (those with fully erupted permanent dentition; see Woodman et al. 2020) were included in our analyses. However, we georeferenced all specimen localities and generated a map using the ggplot2, rnaturalearth, and ggspatial packages in R (R Core Team 2022; Wickham 2016; South 2017; Dunnington 2020) (Fig. 1). Examined specimens are housed in the following museums: American Museum of Natural History (AMNH), New York, NY; Field Museum of Natural History (FMNH), Chicago, IL; and The Natural History Museum (NHMUK), London, UK (Appendix 1; Online Resource 1).
We recorded 19 craniomandibular measurements (Online Resource 1; Sargis et al. 2013, 2014a, b, 2017, 2018; Juman et al. 2021a, b, 2022a, b) from the eight adult specimens using Mitutoyo digital calipers that read to 0.01 mm, with a foot pedal for inputting data. Because some skulls were broken or damaged, we were unable to record all measurements for all individuals. Original measurements (in mm) are provided in Online Resource 1. We conducted all subsequent analyses with the following seven complete variables to maximize sample size: upper toothrow length (UTL), braincase breadth (BB), lambdoid-premaxillary length (LPL), postorbital bar-premaxillary length (PBPL), mandibular height (MH), mandibular condyle width (MCW), and mandibular condyle height (MCH).
Additional data for the same variables from the following seven treeshrew species were assembled from existing datasets: T. belangeri (n = 530; Juman et al. 2022a); T. glis (n = 152; Sargis et al. 2018); T. minor (n = 55; Juman et al. 2022b); T. palawanensis (n = 76; Online Resource 1; Sargis et al. 2014a); T. tana (n = 142; Juman et al. 2021b); Ptilocercus lowii (n = 36; Juman et al. 2021a); and Dendrogale melanura (n = 8; previously unpublished; Online Resource 1).
Multivariate analyses were performed on log-transformed (ln) data in R. We conducted principal component analyses (PCA) on correlation matrices with the “princomp” function and discriminant function analyses (DFA) with the MASS package (Venables and Ripley 2002). Plots were created using ggplot2 (Wickham 2016).
We first investigated morphometric variation between Anathana (n = 8) and a subset of neighboring T. belangeri (defined as west of the Arakan Mountains, longitude < 92.5° E in the south and < 97° E in the north; n = 30) using a PCA of the seven variables listed above. We then performed a DFA of these same two groups and cross-validated our model with a jackknifing procedure. We also investigated shape differences in this subset using a PCA of the same seven variables standardized with a size proxy (calculated as the geometric mean of these measurements for each individual). Next, we conducted a PCA on the overall combined dataset with all eight species included (n = 1,007). Finally, we performed a PCA grouped by population (species or subspecies) within the Anathana sample: A. e. ellioti (n = 1), A. e. “pallida” (n = 5), and A. e. “wroughtoni” (n = 2). We were unable to assess sexual dimorphism within the Anathana sample due to an uneven composition of two females, five males, and one unknown. However, most other treeshrews do not exhibit sexual size dimorphism (Emmons 2000; Woodman et al. 2020; Juman et al. 2021a, b), so we therefore combined males and females in all analyses.
Results
In our PCA of seven skull variables from Anathana and neighboring Tupaia belangeri, principal component (PC) 1 accounts for almost 83% of the variance and has high loadings across all variables (Table 1), suggesting that it represents size. PC2 explains over 7% of the variance and is most influenced by negatively weighted mandibular height variables (Table 1). A bivariate plot of these two components reveals complete separation between the two taxa along PC1, with Anathana plotting in negative morphospace and T. belangeri plotting mostly in positive morphospace (Fig. 2a), suggesting that Anathana has a smaller skull than T. belangeri. A DFA of this sample using the same variables produced a 100% correct classification rate. In our size-corrected version of this PCA, PC1 accounts for over 73% of the variance and represents a contrast between skull length/breadth and mandibular condyle width, while PC2 represents over 20% of the variance and tracks negatively weighted mandibular height variables (Table 1). A plot of these PC scores revealed almost complete separation along PC1 in the reverse order as the original PCA (Fig. 2b), suggesting that Anathana has a longer skull relative to its mandibular condyle width when compared to T. belangeri.
Plots of principal component (PC) scores from principal component analyses of seven craniomandibular variables: a. Plot of Anathana ellioti and neighboring Tupaia belangeri (Table 1); b. Size-corrected plot of A. ellioti and neighboring T. belangeri (Table 1); c. Plot of A. ellioti, T. belangeri (complete dataset), T. glis, T. minor, T. palawanensis, T. tana, Dendrogale melanura, and Ptilocercus lowii with polygons for each genus (Table 2)
We further examined the morphometric distinctiveness of Anathana in another PCA of the same seven skull variables from Anathana, T. belangeri, T. glis, T. minor, T. palawanensis, T. tana, Dendrogale melanura, and Ptilocercus lowii. PC1 is once again a size vector, accounting for over 86% of the variance, while PC2 accounts for almost 8% of the variance and represents negatively weighted mandibular height and condylar width variables (Table 2). A resulting bivariate plot shows Anathana to be completely separated from all other taxa along PC1, with varying degrees of overlap among the other seven taxa (Fig. 2c). This suggests that the Anathana skull is larger than those of T. minor, D. melanura, and P. lowii but smaller than those of T. belangeri, T. glis, T. palawanensis, and T. tana. However, Anathana still falls entirely within the range of variation of Tupaia as a whole (Fig. 2c), even in a size-corrected version of this PCA (not shown).
Finally, we examined possible intraspecific variation within the Anathana sample with a PCA of seven skull variables. PC1 represents over 47% of the variance and has high loadings for cranial variables, suggesting that it tracks cranial size more than mandibular size (Table 3). PC2 accounts for almost 33% of the variance and represents negatively weighted mandibular variables, suggesting that it is inversely correlated with mandibular size (Table 3). A bivariate plot of these two components reveals separation between A. e. “pallida” (n = 5) and A. e. “wroughtoni” (n = 2) along PC1, while the single specimen of A. e. ellioti falls within the polygon of A. e. “pallida” (Fig. 3a). This suggests that A. e. “pallida” and A. e. ellioti have smaller crania than A. e. “wroughtoni”, though our sample sizes for all populations are small.
a. Plot of principal component (PC) scores from a principal component analysis of Anathana ellioti populations, based on seven craniomandibular variables (Table 3); b. Map of India showing localities of museum specimens with species designations recognized by Lyon (1913), including subadults and juveniles not used in analyses
Discussion
Our morphometric analyses suggest that the skull of Anathana is morphometrically distinct from the most proximate T. belangeri specimens on the Indian mainland in both size and shape (Fig. 2a, b). However, in a broader analysis with seven other treeshrew taxa included, Anathana fell within the range of size variation across Tupaia species (Fig. 2c; Table 2). Our results indicate that Anathana is larger than the three smallest species in our analyses (T. minor, D. melanura, and P. lowii) but is smaller than all other members of Tupaia that were included in the study. This is congruent with the original description of the genus, which cited a “shortened rostrum” and “correspondingly short” premaxilla (Lyon 1913: p. 121). The overlap between Anathana and Tupaia in skull size and shape casts doubt on the validity of the former’s generic status, especially given the morphometric distinctiveness of Ptilocercus and Dendrogale (Fig. 2c). Previous molecular research has supported Anathana as the sister taxon to Tupaia (Olson et al. 2005; Roberts et al. 2011). Although the relevant node lacked strong support, it nonetheless remains the best-supported hypothesis adduced to date using modern phylogenetic methods. An updated molecular analysis with more data is needed to help clarify the phylogeny of tupaiids and shed light on ancient patterns of diversification among scandentians.
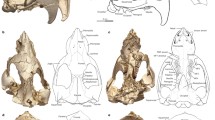
We also conducted a qualitative examination to identify possible diagnostic features that distinguish Anathana from Tupaia. As outlined above, Lyon (1913) described several such differences, including a “relatively short and heavy rostrum” (p. 120) in Anathana (Fig. 4a). One distinguishing feature not noted by Lyon (1913) involves the size and shape of the nuchal crest (Fig. 4). The nuchal crest of Anathana overhangs the supraoccipital (Fig. 4c), whereas this is not the case in Tupaia (Fig. 4d; see also Wible 2011). Furthermore, the shape of this crest differs in the two genera. In Anathana, it is characterized by two lateral convexities, resulting in a bilobate appearance in dorsal view (Fig. 4a), whereas Tupaia exhibits a single rounded convexity (Fig. 4b).
Crania of Anathana ellioti (left column, NHMUK 1850.8.21.17, paratype) and Tupaia belangeri “lepcha” (right column, NHMUK 15.9.1.44, subspecies holotype) in dorsal (a, b) and lateral (c, d) views (from the collections of the Natural History Museum, London). Abbreviation: nc, nuchal crest. Specimens scaled to cranium length. Scale bars equal 5 mm
Within our small Anathana sample (n = 8), the single adult individual of A. ellioti from southeastern India (Fig. 3b) falls inside the range of morphometric variation among (“pallida”) individuals from northeastern India (Fig. 3a). This result is expected based on the fact that the three taxa (formerly species) were originally differentiated from each other based on pelage, with no diagnostic cranial characteristics (Lyon 1913). Lyon (1913: p. 124) specifically noted that “[t]here are no tangible characters by which the skull and teeth of Anathana wroughtoni may be distinguished from those of A. ellioti.” However, the two (“wroughtoni”) specimens from western India (Fig. 3b) are morphometrically distinct from the other six individuals due to their larger crania (Fig. 3a). This might be related to their geographic separation on the opposite side of India from the more eastern populations (Fig. 3b); perhaps a barrier to east-west dispersal, such as the Western Ghats mountain range, could explain the potential distinction of this population. Similarly, east-west rainfall patterns may produce ecophenotypic size variation, as has been observed in other small mammals (Teta et al. 2022). Although we cannot rule out that this western (“wroughtoni”) population may be craniometrically distinct from other Anathana populations, we still follow Helgen (2005) in not recognizing any subspecies due to the small available sample sizes.
The geographic range of A. ellioti spans most of the Indian subcontinent, yet there are fewer Anathana specimens in museum collections than those of T. palawanensis or T. nicobarica, both of which are restricted to small archipelagos. Its rarity in museum collections relative to other scandentians could be a result of elusiveness and apparent sensitivity to anthropogenic disturbance (Hawkins 2018a; Patel et al. 2020). Anathana is currently listed as “Least Concern” on the IUCN Red List of Threatened Species, but populations are thought to be undergoing localized declines and the overall trend is “decreasing” (Molur 2016).
Another possible explanation is the British East India Company’s heavier focus on collecting natural history specimens from northern and eastern colonial territories—rather than South India—by the mid-19th century (Ratcliff 2016). Many of these specimens were added to The Natural History Museum collection in London (Leviton and Aldrich 2000), which today reflects uneven collecting of different treeshrew taxa across former British colonies. The scarcity of Anathana specimens is likely exacerbated by ongoing logistical difficulties associated with collecting specimens—and, more generally, performing wildlife research—in India (Madhusudhan et al. 2006). This species in particular is severely lacking a sufficiently large and evenly distributed sample of museum specimens (Fig. 1), hindering future efforts to study South India’s only treeshrew—a potentially threatened monotypic genus. A larger sample size is necessary to thoroughly investigate the morphological and molecular distinctiveness of Anathana and determine whether it warrants generic status. Our study highlights the critical need for continued specimen collection to examine, delineate, and ultimately conserve Indian mammal species.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its Supplementary Materials.
References
Corbet GB, Hill JE (1992) The Mammals of the Indomalayan Region: A Systematic Review. Oxford University Press, Oxford
Dunnington D (2020) ggspatial: Spatial Data Framework for ggplot2. R package version 1.1.4
Emmons LH (2000) Tupai: a Field Study of Bornean Treeshrews. University of California Press, Berkeley
Fiedler W (1956) Ubersicht über das System der Primates. Primatologia 1:1-266
Hawkins MT (2018a) Family Ptilocercidae (Pen-tailed Treeshrew). In: DE Wilson, RA Mittermeier (eds) Handbook of the Mammals of the World. Vol. 8. Insectivores, Sloths and Colugos. Lynx Edicions, Barcelona, pp 236-241
Hawkins MT (2018b) Family Tupaiidae (Treeshrews). In: DE Wilson, RA Mittermeier (eds) Handbook of the Mammals of the World. Vol. 8. Insectivores, Sloths and Colugos. Lynx Edicions, Barcelona, pp 242-269
Helgen KM (2005) Order Scandentia. In: DE Wilson, DM Reeder (eds) Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd edn. Johns Hopkins University Press, Baltimore, pp 104-109
Juman MM, Millien V, Olson LE, Sargis EJ (2022a) Recent and rapid ecogeographical rule reversals in Northern Treeshrews. Sci Rep 12:19689. https://doi.org/10.1038/s41598-022-23774-w
Juman MM, Olson LE, Sargis EJ (2021a) Skeletal variation and taxonomic boundaries in the Pen-tailed Treeshrew (Scandentia: Ptilocercidae; Ptilocercus lowii Gray, 1848). J Mamm Evol 28:1193-1203. https://doi.org/10.1007/s10914-021-09556-7
Juman MM, Woodman N, Miller-Murthy A, Olson LE, Sargis EJ (2022b) Taxonomic boundaries in Lesser Treeshrews (Scandentia, Tupaiidae: Tupaia minor). J Mammal 103:1431-1440. https://doi.org/10.1093/jmammal/gyac080
Juman MM, Woodman N, Olson LE, Sargis EJ (2021b) Ecogeographic variation and taxonomic boundaries in Large Treeshrews (Scandentia, Tupaiidae: Tupaia tana Raffles, 1821) from Southeast Asia. J Mammal 102:1054-1066. https://doi.org/10.1093/jmammal/gyab059
Leviton AE, Aldrich ML (2000) India: A case study of natural history in a colonial setting. In: MT Ghiselin, AE Leviton (eds) Cultures and Institutions of Natural History: Essays in the History and Philosophy of Science. California Academy of Sciences, San Francisco, pp 51-80
Lyon MW (1913) Treeshrews: an account of the mammalian family Tupaiidae. Proc US Natl Mus 45:1-188
Madhusudhan MD, Shanker K, Kumar A et al. (2006) Science in the wilderness: The predicament of scientific research in India’s wildlife reserves. Curr Sci 91:1015-1019
Molur S (2016) Anathana ellioti. The IUCN Red List of Threatened Species 2016:e.T39593A22278758. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T39593A22278758.en. Accessed 31 July 2023
Olson LE, Sargis EJ, Martin RD (2005) Intraordinal phylogenetics of treeshrews (Mammalia: Scandentia) based on evidence from the mitochondrial 12S rRNA gene. Mol Phylogenet Evol 35:656-673. https://doi.org/10.1016/j.ympev.2005.01.005
Patel K, Vyas A, Naik V, Patel H (2020) On the occurrence of Madras Tree Shrew Anathana ellioti (Waterhouse) (Scandentia: Tupaiidae) from Gujarat State, India. Trop Nat Hist 20:111-115
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org/
Ratcliff J (2016) The East India Company, the Company’s museum, and the political economy of natural history in the early nineteenth century. Isis 107:495-517. https://doi.org/10.1086/688433
Roberts TE, Lanier HC, Sargis EJ, Olson LE (2011) Molecular phylogeny of treeshrews (Mammalia: Scandentia) and the timescale of diversification in Southeast Asia. Mol Phylogenet Evol 60:358-372. https://doi.org/10.1016/j.ympev.2011.04.021
Sargis EJ (2004) New views on tree shrews: The role of tupaiids in primate supraordinal relationships. Evol Anthropol 13:56-66. https://doi.org/10.1002/evan.10131
Sargis EJ (2020) Scandentia. In: K de Queiroz, PD Cantino, JA Gauthier (eds) Phylonyms: A Companion to the PhyloCode. CRC Press, Boca Raton, pp 899-902
Sargis EJ, Campbell KK, Olson LE (2014a) Taxonomic boundaries and craniometric variation in the treeshrews (Scandentia, Tupaiidae) from the Palawan Faunal Region. J Mamm Evol 21:111-123. https://doi.org/10.1007/s10914-013-9229-2
Sargis EJ, Millien V, Woodman N, Olson LE (2018) Rule reversal: Ecogeographical patterns of body size variation in the common treeshrew (Mammalia, Scandentia). Ecol Evol 8:1634-1645. https://doi.org/10.1002/ece3.3682
Sargis EJ, Woodman N, Morningstar NC, Reese AT, Olson LE (2013) Morphological distinctiveness of Javan Tupaia hypochrysa (Scandentia, Tupaiidae). J Mammal 94:938-947. https://doi.org/10.1644/13-MAMM-A-042.1
Sargis EJ, Woodman N, Morningstar NC, Reese AT, Olson LE (2014b) Island history affects faunal composition: the treeshrews (Mammalia: Scandentia: Tupaiidae) from the Mentawai and Batu Islands, Indonesia. Biol J Linn Soc 111:290-304. https://doi.org/10.1111/bij.12195
Sargis EJ, Woodman N, Morningstar NC, Bell TN, Olson LE (2017) Skeletal variation and taxonomic boundaries among mainland and island populations of the common treeshrew (Mammalia: Scandentia: Tupaiidae). Biol J Linn Soc 120:286-312. https://doi.org/10.1111/bij.12876
Sehgal RK, Singh AP, Gilbert CC, Patel BA, Campisano CJ, Selig KR, Patnaik R, Singh NP (2022) A new genus of treeshrew and other micromammals from the middle Miocene hominoid locality of Ramnagar, Udhampur District, Jammu and Kashmir, India. J Paleontol 96:1318-1335. https://doi.org/10.1017/jpa.2022.41
South A (2017) rnaturalearth: World Map Data from Natural Earth. R package version 0.1.0
Teta P, de la Sancha NU, D'Elía G, Patterson BD (2022) Andean rain shadow effect drives phenotypic variation in a widely distributed austral rodent. J Biogeogr 49:1767-1778. https://doi.org/10.1111/jbi.14468
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, New York
Verma K (1965) Notes on the biology and anatomy of the Indian Tree-Shrew, Anathana wroughtoni. Mammalia 29:289-330. https://doi.org/10.1515/mamm.1965.29.3.289
Waterhouse G (1850) Description of a new species of Tupaia discovered in continental India by Walter Elliot Esq. Proc Zool Soc Lond 1849:106-108.
Wible JR (2011) On the treeshrew skull (Mammalia, Placentalia, Scandentia). Ann Carnegie Mus 79:149-230. https://doi.org/10.2992/007.079.0301
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Woodman N, Miller-Murthy A, Olson LE, Sargis EJ (2020) Coming of age: morphometric variation in the hand skeletons of juvenile and adult Lesser Treeshrews (Scandentia: Tupaiidae: Tupaia minor Günther, 1876). J Mammal 101:1151-1164. https://doi.org/10.1093/jmammal/gyaa056
Acknowledgements
MMJ thanks Usha Kutty for assistance with georeferencing colonial locality names. We thank the following curators, collection managers, and museums for access to the collections in their care: R. Voss, N. Duncan, and M. Surovy (AMNH); R. Portela-Miguez, L. Tomsett, and P.D. Jenkins (NHMUK); J. Chupasko and M. Rutzmoser (MCZ); and L.R. Heaney, A. Ferguson, and J.D. Phelps (FMNH). We thank the associate editor Rex Mitchell and two anonymous reviewers for valuable comments that greatly improved the manuscript.
Funding
This study was funded by National Science Foundation grants DEB-0542532 and DEB-0542725 and an Alaska EPSCoR grant to EJS and LEO. MMJ is supported by a Gates Cambridge Scholarship enabled by grant OPP1144 from the Bill & Melinda Gates Foundation. LEO and MMJ were also supported in part by a generous donation from the Jay Pritzker Foundation.
Author information
Authors and Affiliations
Contributions
All authors designed the study. EJS and MMJ collected the data. LEO developed the data-collection workflow, and MMJ, LEO, and EJS curated the data. MMJ led the analyses and wrote the paper with substantial input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Online Resource 1 (XLSX 128 kb)
Descriptions of skull measurements and original data from the Anathana ellioti, Dendrogale melanura, and Tupaia palawanensis specimens used in this study.
Appendix 1 Specimens examined
Appendix 1 Specimens examined
Specimens from the following institutions were included in this study: American Museum of Natural History (AMNH), New York, NY; Field Museum of Natural History (FMNH), Chicago, IL; and The Natural History Museum (NHMUK), London, UK. Non-adult specimens omitted from analyses but included in maps are indicated with an asterisk.
Anathana ellioti ellioti (n = 4)—India: Andhra Pradesh: hills between Cuddapah [Kadapa] and Nellore, near Madras [Chennai] (NHMUK 1850.8.21.17—paratype; NHMUK 1850.1.21.7*—paratype); Palkonda Hills (FMNH 82603*); Shevaroy Hills (NHMUK 91.10.7.49*).
Anathana ellioti “pallida” (n = 6)—India: Manbhum [Purulia] (NHMUK 66.12.28.2—holotype); Bengal, Palamai [Palamu] (NHMUK 78.2527); Madhya Pradesh (FMNH 91265); Bihar, Nawadeh [Nawadih] (AMNH M-150086); Central Provinces [Chhattisgarh]: Nawpara [Nawapara] (AMNH M-171125); Rajpur [Raipur] (NHMUK 85.8.1.97*).
Anathana ellioti “wroughtoni” (n = 2)—India: Gujarat: Surat, Mandvi (NHMUK 96.11.7.1—holotype); Dangs, Maheskatri (NHMUK 96.11.7.2).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juman, M.M., Olson, L.E. & Sargis, E.J. Craniometric variation and taxonomic boundaries in the Madras Treeshrew (Scandentia, Tupaiidae: Anathana ellioti [Waterhouse, 1850]) from India. J Mammal Evol 31, 4 (2024). https://doi.org/10.1007/s10914-023-09694-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10914-023-09694-0