Abstract
A new schizotheriine mandible from the early Miocene Xianshuihe Formation in Lanzhou Basin, Northwest China is described here. Compared with other contemporaneous taxa, the lower jaw is most similar to Borissiakia betpakdalensis from Kazakhstan based on mandibular morphology and dental features, except for a much smaller body size which is hardly explained by intraspecific variation and suggests it represents a smaller body-sized species of Borissiakia. The type specimen of Phyllotillon huangheensis, from the same strata of the Lanzhou Basin, shares characters with the new specimen in the lower cheek teeth and the tapered morphology of the anterior horizontal ramus. Differences between both are reflected in the height of the ramus, especially the level of the symphysis, which may be an expression of sexual dimorphism. Therefore, schizotheriine mandibles from the early Miocene of Lanzhou Basin are regarded as the same taxon, and both are recognized as Borissiakia huangheensis. The occurrence of a chalicothere and other large perissodactyls may suggest that a certain amount of open woodland was distributed across the basin and that the paleoclimate might have been more humid during the early Miocene.
Similar content being viewed by others
Introduction
Chalicotheriidae is a family of peculiar Perissodactyla with claws on their digits instead of the typical hooves of ungulates. The unusual association of claws (which are generally present in carnivores among large mammals) with a herbivorous dentition pattern differentiates these bizarre taxa from any other living or extinct perissodactyls (i.e., horses, tapirs, rhinoceroses, and brontotheres), and has drawn the attention of paleontologists since chalicotheriids were first known. The family Chalicotheriidae is divided into two subfamilies (Chalicotheriinae and Schizotheriinae) primarily based on dental and postcranial characters of the two groups (Coombs 1978a, 1989). At present, Winamia, Chalicotherium, Kalimantsia, Anisodon, Nestoritherium, and Hesperotherium are assigned to Chalicotheriinae (Chen et al. 2012); Schizotheriinae includes seven genera: Schizotherium, Borissiakia, Phyllotillon, Moropus, Metaschizotherium, Tylocephalonyx, and Ancylotherium (Fahlke and Coombs 2009). Compared with Chalicotheriinae, the subfamily Schizotheriinae exhibits a less robust mandible, shorter mandibular symphysis, and more derived cheek teeth, but the postcranial skeletons remain more conservative and primitive than in the members of Chalicotheriinae (Butler 1965; Coombs 1978a, 1989; Zapfe 1979; Coombs and Cote 2010).
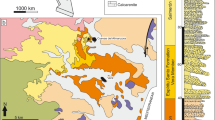
The most primitive Chalicotheriidae are represented by the schizotheriine Schizotherium from the Oligocene of Eurasia (China, Mongolia, Kazakhstan, and Western Europe), then the family reached its peak of taxonomic diversity during the Miocene, widespread in Eurasia, Africa, North America and even migrating to the Panama Canal Basin of Central America (Fig. 1) (Forster-Cooper 1920; Colbert 1934; Borissiak 1946; Butler 1965; Coombs 1978a, b, 1989, 2009; Heissig 1999; Coombs et al. 2001; Coombs et al. 2004; Chen 2008; Chavasseau et al. 2010; Coombs and Hunt 2015; Wood and Ridgwell 2015). However, in Asia, chalicothere fossils are extremely rare during the early Miocene, and there are only a few reports from Pakistan (Baluchistan), Kazakhstan (Betpak-dala), and China (Lanzhou), whereas abundant chalicothere fossils have been discovered in North America (Moropus and Tylocephalonyx) and Europe (Metaschizotherium, Phyllotillon and Moropus). In the early Miocene of East Asia, only a schizotheriine mandible from the Lanzhou Basin has been reported and described as Phyllotillon huangheensis; two other heavily broken skull fragments have been identified as Chalicotheriinae gen. et sp. indet. (Qiu et al. 1998). In addition, Handa and Kawabe (2016) reported a femur that was previously assigned to Chilotherium pugnator from the lower Miocene Hiramaki Formation of the Mizunami Group in Gifu Prefecture of Central Japan and re-identified it as a schizotheriine chalicothere of uncertain genus and species. In fact, P. huangheensis represents the only well-documented early Miocene chalicothere from China, and its taxonomic designation is still controversial. The main objective of this paper is to report a new chalicothere material from the early Miocene Xianshuihe Formation of the Lanzhou Basin, China and to figure out its taxonomic relationship with the contemporaneous chalicotheres of the area, contributing to the knowledge of chalicothere evolution in the early Miocene of China.
Geological Setting
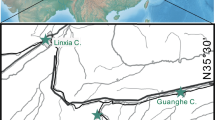
The Lanzhou Basin belongs to the integrated Longzhong Basin, which is located in the connecting area between the northeastern Tibetan Plateau and the Loess Plateau as well as the transitional zone of Asian monsoon-influenced region and the arid inland area in Northwest China (Fig. 2a, b). Although the subbasin is not large (~ 300 km2), in it developed thick and continuous fluvio-delta-lacustrine sedimentary sequences spanning the Paleogene to Neogene, containing Oligocene to Miocene mammal assemblages (Qiu et al. 2013). From older to younger, the Paleocene to Miocene strata include the early Eocene Xiliugou Formation, which unconformably overlies the lower Cretaceous Hekou Group, the late early Eocene to early Oligocene Yehucheng Formation, and the late early Oligocene to Miocene Xianshuihe Formation (Fig. 2b, c) (Zhai and Cai 1984; Yue et al. 2000).
The study area, Tertiary strata, and stratigraphic column of the Middle Member of the Xianshuihe Formation. a-b location of the study area (China mainland map from China National Bureau of Surveying and Mapping Geographical Information, Ministry of Natural Resources of the P.R.C); c outcrops of the Tertiary strata in the Lanzhou Basin with localities of mammal fossils; d early Miocene stratigraphy of the Lanzhou Basin, showing the stratum containing Borissiakia
The Xianshuihe Formation is the most important fossiliferous stratum for it produces all the known local mammalian faunas in the basin. It mainly consists of thick brownish-red silty mudstone interbedded with sandstone which is subdivided into the Lower, Middle and Upper Members based on the sedimentary cycle (Qiu et al. 1997). Based on the combined lithology, sedimentary structures, and facies fossils, we infer that the majority of these sediments were deposited in delta front and shallow-lake environments. The Middle Member of the Xianshuihe Formation is about 200 ~ 225 m thick and has produced the Zhangjiaping and Duitinggou local faunas (Qiu et al. 1997; Yue et al. 2000). The bottom of the Middle Member consists of white fine-grained conglomerate and coarse sandstone with well-developed cross-bedding, sedimented in the distributary channel in a delta plain environment, indicating stronger hydrodynamic conditions (Fig. 2d). The thick white sandstone is widespread in the basin and well exposed in several sections, such as Duitinggou, Huangyangtou, and Dahonggou, and has produced the Zhangjiaping local fauna which contains more than 30 taxa of small and large mammals (Qiu et al. 2013), including the Schizotheriinae Phyllotillon huangheensis (Qiu et al. 1998) and the new chalicothere mandible described in this paper. The magnetostratigraphic age of the Zhangjiaping fauna is roughly 22 ~ 21 Ma (Qiu et al. 2013) or 21 ~ 20 Ma (Zhang 2015), and belongs to the Xiejian stage (Chinese mammal ages). Thus, the schizotheriine material is older than the schizotheriine femur from Japan which is estimated to be 18.4 ~ 18.0 Ma (Handa and Kawabe 2016).
Material and Methods
Dental terminology follows Coombs (1978a), Fahlke and Coombs (2009) (Fig. 3).
Anatomical abbreviations. i, lower incisor; p, lower premolar; m, lower molar.
Institutional acronyms. AMNH, American Museum of Natural History, New York City, USA; BMNH, British Museum of Natural History, London, UK; BSPG, Bayerische Staatssammlung für Paläontologie und historische Geologie, Munich, Germany; CM, Carnegie Museum of Natural History, Pittsburgh, USA; GVL, Gansu Provincial Museum, Lanzhou, China; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; LSUMG, Louisiana State University Museum of Geoscience, Baton Rouge, USA; NWU, Northwest University, Xi’an, China; UF, Florida Museum of Natural History, Gainesville, USA; ZD, Zdeněk Dvořák Collection at the National Museum, Prague, Czech Republic.
Systematic Paleontology
Order Perissodactyla Owen, 1848
Family Chalicotheriidae Gill, 1872
Subfamily Schizotheriinae Holland and Peterson, 1914
Genus Borissiakia Butler, 1965
Borissiakia huangheensis (Qiu, Wang and Xie, 1998).
Material. NWU V 1542, a left mandible belonging to an adult individual, preserving diastema, roots of p2-m1 and m2-m3 crowns; symphysis and rostrum broken (Fig. 4).
Locality and horizon. Huangyangtou village, 30 km northwest of Lanzhou City, Gansu Province; at the bottom of the Middle Member of Xianshuihe Formation, early Miocene.
Emended diagnosis of Borissiakia (revised from Borissiak 1946; Coombs 1989). Schizotheriine chalicotheriid of small to medium size; mandible slender with straight borders, anterior end of the ramus tapered rather than rounded; molars elongated and cingula less developed except posterolingual cingulum on the hypocone of M3; tarsals and metatarsals long and slender; cuboid connecting with distal surface of astragalus; carpals and metacarpal slender but deep.
Emended diagnosis of B. huangheensis (revised from Qiu et al. 1998). A small-sized species of Borissiakia, mandible slender with straight lower borders; symphysis short, anterior ramus tapered; lower molars elongated, m3 larger than m2; lophids straight or slightly curved, metaconid and metastylid separated, without hypoconulid; lingual cingula rarely developed.
Description. The left mandible is slender with a total length of 289 mm. In labial view, the lower border of the ramus is extremely straight and horizontal, only presenting a weak bulge below the posterior part of the symphysis. The lower border between the p2 and symphysis forms a sharp ridge and is slightly curved inwards. In occlusal view, the labial side of the horizontal ramus is flat whereas the lingual side below the cheek teeth, especially below the molar row forms an inward convexity with a width of 27.8 mm. The height of the horizontal ramus decreases anteriorly and tapers distinctly at the front end just as the measurements show: with a depth of 57 mm posterior to m3, 37.6 mm anterior to m1, 27.8 mm anterior to p2, and 27.1 mm posterior to the symphysis. Significantly, the height of the mandible does not decrease sharply from p2 forwards and the level of the diastema is just slightly lower than the p2 alveolar plane, without an obvious descent anterior to the premolars. The mandibular symphysis is remarkably short (39.8 mm) and extends posteriorly close to the p2 (only 10.2 mm). A tiny round pit with smooth wall on the front edge of the jaw is presumably the remnant alveolus of i3, which is only 39 mm away from p2. Therefore, the diastema is shorter than the premolar row (49.5 mm). There is an oval mental foramen located below the diastema with a maximum diameter of 10 mm that is much closer to i3 than p2.
The anterior lower cheek teeth are poorly preserved. It is difficult to describe the dental morphology and features of the chewing facets of the broken premolars and m1. Nevertheless, the remnants of the roots are still helpful to estimate the size of the fractured teeth. Compared with molars, the premolars are much shorter and narrower and the lower premolar row is just 49.5 mm long, less than half of the lower molar row. Measurements of the cheek tooth row are given in Table 1.
From p2 to p4, the size of the lower premolars increases gradually. The p2 is extremely small and the cross section of the crown base is triangular. The CT-scanning image reveals that the p2, as well as the other lower cheek teeth, develop double roots (Fig. 5). The p3 is slightly larger than p2, and the cross section of its crown base is rectangular. Its anterior root is close to the posterior root of the p2, while there is a short gap between the roots of p3 and p4. The p4 is larger than p3 but is much smaller than the lower molars. Judged from the root remains, the length of the trigonid is almost the same as the talonid, but the latter is slightly wider.
The anterolabial enamel of m2 is broken and the talonid is distinctly longer and wider than the trigonid. Due to heavy abrasion, the paraconid becomes rounded and is the lowest cuspid; the trigonid basin nearly disappears, just a tiny pit left. The breakage of the metaconid and metastylid makes it difficult to determine the development and morphology of both cuspids. Of all cuspids, the paraconid is the weakest and lowest. The paralophid and protolophid are slightly curved and meet at the protoconid in a blunt angle, forming a U-shaped trigonid. The less curved metalophid and hypolophid join at the hypoconid in a blunt-tipped acute angle. The labial cingulum is extremely weak; only the posterior cingulum tilts up from the posterolabial side and forms a small bulge below the entoconid; almost no cingulum is observed on the lingual side.
The m3 is well preserved and less worn than m2. Its occlusion with the upper molar forms a deep pit on the trigonid and all the anterior cuspids protrude upwards distinctly. The talonid has almost the same width as the trigonid but the former is longer; the labial valley between them is extremely deep with an inverted “V” shape. The paraconid is the lowest cuspid; the entoconid is strong, higher than the paraconid, protoconid and hypoconid. The tallest cuspid, the metaconid, though broken, is presumed to be separated from the metastylid by a shallow notch at the tip of the lingual side. The paralophid is slightly more curved than other lophids, nearly perpendicular to the tooth row; the protolophid is long and straight, extending anteriorly from the metaconid to the protoconid. Both lophids of the trigonid meet at a blunt-tipped acute angle at the protoconid. The talonid is less worn and slightly higher than the trigonid. As the longest lophid, the metalophid is fairly straight and oblique posteriorly while the hypolophid is also straight but shorter. The metalophid and hypolophid form a V-shaped talonid with an acute angle at the hypoconid. Only weak labial and posterior cingula can be observed and there is almost no lingual cingulum just as in the m2; hypoconulid is also absent on m3.
Comparisons
Comparison with Chalicotheriinae
Mandibular and dental features of Chalicotheriinae, such as the relatively robust jaws, longer mandibular symphysis, the conservative dentition with low-crowned cheek teeth and preservation of lower canines are fundamentally different from those of Schizotheriinae (Butler 1965; Zapfe 1979; Coombs 1989; Coombs and Cote 2010). In contrast, schizotheriine chalicotheres usually develop less robust jaws and show a lower width-height ratio of the horizontal ramus, for example, only 39.5% at m1 in Schizotherium cf. S. avitum (Liu and Zhang 2012) and just 35.1% in P. huangheensis (IVPP V 9959) (Qiu et al. 1998). Although the Huangyangtou chalicothere has a higher ratio of 49% under m1/m2 than the above schizotheriine taxa, the value is still much lower than in some chalicotheriines such as Chalicotherium brevirostris (56% ~ 65%) (Liu and Zhang 2012) and “Chalicotherium” salinum (51% at m1) (Chavasseau et al. 2010), suggesting a much more slender mandible.
Other important features of NWU V 1542 are the absence of the lower canine and a short diastema, which distinguishes it from chalicotheriines. One of them is Winamia rusingensis, a primitive chalicotheriine from the early Miocene of East Africa that was previously referred to Chalicotherium rusingensis (Butler 1965) and later to Butleria rusingensis (Bonis et al. 1995). Since Coombs and Cote (2010), it has been realized that the genus name Butleria is a junior homonym of another Butleria named for a genus of butterfly in 1871, so Pickford (2020) replaced it with the new genus name Winamia. W. rusingensis possesses a relatively long symphysis (63 mm) and lower canines (Butler 1965), but its diastema is somewhat shorter (27.5 mm). Its cheek teeth and lower jaw are also smaller, regarded as primitive features (Fig. 6). “Chalicotherium” pilgrimi from the Oligocene-early Miocene Bugti fauna of Pakistan, whose lower jaw is still not known, may resemble W. rusingensis in many ways (Coombs and Cote 2010). The slender mandible, short symphysis, and the lack of lower canines support the attribution of the Huangyangtou chalicothere to the subfamily Schizotheriinae.
Measurements of m2 and m3 among Oligocene-early Miocene chalicotheriids: Winamia rusingensis (F 3608, 1782.5) from Butler (1965); Schizotherium ordosium (GVL 8710, IVPP V 2401.3) from Qiu et al. (1998); Phyllotillon naricus (BMNH M 12,164 A-B, BMNH M 12,164 A-C) from Forster-Cooper (1920), Coombs (2009); Phyllotillon schlosseri from Heissig and Feifar (2013); Moropus elatus (CM 1914, CM 1755, CM1759) from Holland and Peterson (1914), Coombs (1973, 1978a, b); M. cf. oregonensis (UF 180,233, LSUMG-V 2489) from Coombs et al. (2001); Metaschizotherium wetzleri (1867 XII 5) from von Koenigswald (1932), Heissig and Fejfar (2013); Borissiakia betpakdalensis calculated from Borissiak (1946); Borissiakia huangheensis (IVPP V 9959) from Qiu et al. (1998)
Comparison with Schizotheriinae
Coombs (1978a, b, 1979, 1989, 2009) re-diagnosed Schizotheriinae and the relationships among members based on cranial and dental features which serve as a basis for the systematics of schizotheriines here. The subfamily contains Schizotherium, Borissiakia, Phyllotillon, Moropus, Tylocephalonyx, Metaschizotherium and Ancylotherium spanning from the Oligocene to Pleistocene. Moropus had a primarily North American distribution but has also been identified from the early Miocene of Eurasia, while Metaschizotherium and Tylocephalonyx were restricted to Europe and North America respectively (Coombs 1978a, 1989, 2009; Fahlke and Coombs 2009). The latest surviving genus of the subfamily, Ancylotherium, will not be discussed in the paper as it occurred in the Old World during the late Miocene and Pleistocene.
Comparison with Schizotherium ordosium
Schizotherium is the most primitive genus of the Schizotheriinae with small body size, found in Oligocene deposits throughout Eurasia, with S. ordosium being the largest species (Coombs 1978b, 1989). As the mandible of Schizotherium is poorly known, teeth provide important evidence for comparison with other taxa. Firstly, the Huangyangtou chalicothere differs from S. ordosium in its larger size, which can be judged from the measurements of the lower cheek teeth. The m2 of S. ordosium is smaller (29.4 ~ 32 mm × 16.3 ~ 17.5 mm) than those of NWU V 1542 (33.2 mm × 20.17 mm), and the m3 (33 ~ 36 mm) is only 72 ~ 78% as long as the latter (45.8 mm), suggesting that S. ordosium and other species of Schizotherium had a smaller size than NWU V 1542 (Fig. 6). Furthermore, one of the important dental features is that Schizotherium has begun to develop elongated molars, but they seem in S. ordosium less elongated compared with the later schizotheriines (Coombs 1978b, 1989). The m3 width/length proportion of S. ordosium (47% ~ 48%) is higher than in NWU V 1542 (44%). Besides, the molar row of the former is relatively shorter (Table 1) and the premolar row/molar row ratio is above 50% while that of NWU V 1542 is just 48%, less than half the length of the molar row, further suggesting that the latter develops more elongated molars than the earlier S. ordosium.
Another important morphological difference is that S. ordosium still preserves a well-developed hypoconulid on m3, a primitive character among the schizotheriines. In NWU V 1542, the hypoconulid of the m3 has completely disappeared. Thus, the larger body size, elongation of lower molars, and lack of hypoconulid on m3 in the Huangyangtou chalicothere suggests a more derived taxon than S. ordosium.
Comparison with Borissiakia betpakdalensis
Borissiakia betpakdalensis is the type species of the genus, originally described as Moropus betpakdalensis (Flerov 1938) and then Phyllotillon betpakdalensis (Borissiak 1946), before the genus Borissiakia was established by Butler (1965). Comparatively rich material such as the incomplete skull, lower jaws and postcranial elements were found in the thick fine-grained sandstone interbedded with gravels exposed in the Golodnaya steppe (Betpak-dala), southern Kazakhstan; the age is late Oligocene to early Miocene.
The primary mandibular and dental features of the lower mandible of B. betpakdalensis described by Borissiak (1946) are summarized here: 1) the mandible is slender with straight lower and upper borders except a convexity under the molars and symphysis; 2) the upper border of the mandible anterior to the premolars is sharp and slightly lower than the alveolar plane; 3) the lower molars, especially m3, are strongly elongated and the talonid is longer than the trigonid, with a less deep but wide trigonid basin and talonid basin; 4) metaconid and metastylid are separated at the tip, the hypoconid oblique posteriorly; 5) paralophid is slightly curved and other posterior lophids are straight; 6) the labial and lingual cingulum are weak or barely developed on the anterior and posterior sides.
Based on Borissiak’s (1946) description, it is clear that B. betpakdalensis and NWU V 1542 share common features of mandibular morphology, especially the slender ramus, the straight lower border, weak lowering of the mandibular height at the level of the diastema, and tapered anterior end of ramus. Both mandibles develop a convexity on the lingual side below the lower molar row. In addition, typical dental characters, such as the elongation of lower molars, larger size of m3, longer talonids of molars can also be observed in both taxa. Therefore, it is reasonable to attribute the Huangyangtou chalicothere to Borissiakia.
However, the most obvious difference between NWU V 1542 and B. betpakdalensis is the body size. Borissiak (1946: Pl. 1: Fig. 1) described the largest fragment of a right lower jaw of B. betpakdalensis, which can be readily compared with the Huangyangtou mandible. The total length of this lower jaw of B. betpakdalensis is 385 mm, and the height of the ramus is 67 mm at the posterior border of m3, 55 mm at the anterior border of m1, and 35 mm at the symphysis. Borissiak (1946) mentioned another, smaller lower mandible fragment and the height of the corresponding positions is 60 mm, 52 mm, and 30 mm, respectively. In comparison, the Huangyangtou mandible is only 289 mm in length, and the height of the horizontal ramus at the same positions is only 56.7 mm, 37.9 mm, and 27.8 mm, much smaller than those of B. betpakdalensis (Fig. 7). Furthermore, lower molar sizes of both taxa are totally different (Fig. 6). Although Borissiak (1946) did not give the measurements of lower molars, we calculated the size according to the scale in the image of the mandible. The results show that the molar row of the larger individual discussed above is 145 mm long and the premolar row is estimated at 63 mm (calculated by the scale); the molar row of the smaller individual is 117 mm, all of which are longer than the Huangyangtou chalicothere. Consequently, it is inferred that B. betpakdalensis has a much larger body size than NWU V 1542 based on the different sizes of the mandible and cheek teeth. For B. betpakdalensis, the larger and smaller dimensions may reflect different sexes because sexual dimorphism is rather common among chalicotheres (Coombs 1975).
Another important point is that the larger mandible in fact represents a juvenile individual because the preserved lower molars are less worn (e.g., the metaconid and metastylid still much higher above the chewing surface, the complete lophids, the almost unworn trigonid and talonid basins). Considering the heavily worn lower molars, however, NWU V 1542 is much older than the larger mandibles of B. betpakdalensis, which actually represent two individuals at different developmental stages, so it is impossible that the size variation is caused by different growth ages. Therefore, the different body sizes of B. betpakdalensis and NWU V 1542 is unlikely related to sexual dimorphism or other factors even though wide intraspecific ranges of variation are quite common in schizotheriines (Coombs 1975). Consequently, the Huangyangtou chalicothere probably represents another, smaller species of Borissiakia.
Comparison with Phyllotillon naricus and Phyllotillon schlosseri
Phyllotillon naricus is the type species of this genus from the early Miocene of Bugti, Pakistan (Forster-Cooper 1920). Due to the scarcity of fossils, the taxonomy of Phyllotillon is still controversial. Coombs (1973, 1974, 1989, 2009) and Fahlke and Coombs (2009) suggested that the genus Phyllotillon be restricted to the Bugti collections from Pakistan until better material is found. However, Heissig and Fejfar (2013) attributed the materials from the Chomutov Basin (Czech Republic) to a new species P. schlosseri and regarded Phyllotillon as a valid genus for obvious differences with Moropus.
Both P. naricus and P. schlosseri have straight lower mandible borders, the short diastema with a smooth lowering anterior of p2, features that are similar to the Huangyangtou chalicothere. Besides, the lower molars of P. naricus resemble those of NWU V 1542 in having a larger m3 with well-developed metastylid and relatively straight lophids. The obvious difference, however, is that the front part of the mandible in P. naricus and P. schlosseri are rounded, whereas it tapers more and turns sharply in both NWU V 1542 and IVPP V 9959. Apart from that, P. naricus develops certain lingual cingula at the opening of the trigonid basin and talonid basin of the lower molars (at least visible in some specimens), which are not visible in the Huangyangtou chalicothere. P. schlosseri shows even more pronounced differences with the Huangyangtou material: the m3 of P. schlosseri is shorter than the m2 while NWU V 1542 develops elongated lower molars with the m3 being obviously larger than the m2. Moreover, in P. schlosseri the symphysis extends posteriorly to the middle of p2, whereas it ends 10.2 mm anterior to p2 in NWU V 1542. Besides, according to Heissig and Fejfar (2013), the ramus of P. schlosseri under p4/m1 and p3 is higher than in the Huangyangtou mandible (Fig. 7). The number and location of mental foramina also varies in both taxa: P. schlosseri develops two mental foramina with the larger one located in front of p2 and the smaller one under the alveoli of p2 and p3, but there is only one mental foramen in the Huangyangtou chalicothere, which is closer to the alveolus of i3. The position of the mental foramina can be variable and may not be regarded as a diagnostic feature. Based on the discussion above, the Huangyangtou chalicothere is clearly different from both species of Phyllotillon.
Comparison with Phyllotillon huangheensis
P. huangheensis was established by Qiu et al. (1998) based on a comparatively complete left mandible (IVPP V 9959), excavated from the white sandstone of the early Miocene Xianshuihe Formation in the Taowan village, Lanzhou Basin. It is the first report of an early Miocene schizotheriine chalicothere in China or even East Asia. There are also other opinions on its taxonomic placement. For example, Chen, (personal communication) re-diagnosed the mandible, considered it to have more similarities with mandibles of Moropus, and therefore attributed it to Moropus huangheensis. In any case, V 9959 is an important comparative specimen in the study of early Neogene Schizotheriinae in China.
Just like B. betpakdalensis, both mandibles from the Lanzhou Basin have straight lower borders and short and slender symphyses (about 40 mm long), with the ramus tapering more than the rounded anterior ends of mandibles in P. naricus and P. schlosseri. NWU V 1542 and V 9959 share more similarities on the lower cheek teeth. The premolar row and molar row of V 9959 are just slightly longer than those of NWU V 1542 and both develop elongated lower molars with the premolar row/molar row ratio less than 50%; m3 is larger than m2 (Fig. 6, Table 1). Although the lower molars of V 9959 are slightly larger, the size differences certainly fall within the range of intraspecific variation of the taxon. In regard to the dental morphology of both mandibles: the talonid is longer and broader than the trigonid, with some labial and posterior cingula on m2 and m3; the lophids on the talonid are fairly straight, and the paralophid is slightly curved; and the metaconid and metastylid of m3 are separated at the tip. In occlusal view, the lower cheek teeth of V 9959 are less worn with intact trigonid and talonid basins of m2 and m3, indicating that it represents a younger individual than the Huangyangtou chalicothere.
The two mandibles differ in morphology. Firstly, V 9959 develops a higher but thinner ramus; its length of the horizontal ramus is about 300 mm, longer than Huangyangtou chalicothere. The height of the horizontal ramus decreases anteriorly with 61 mm posterior to m3, 55.5 mm under m1/m2 and 41.6 mm anterior to p2. In occlusal view, the horizontal ramus is as wide as the cheek tooth row and does not show an obvious convexity on the lingual side. In comparison, NWU V 1542 has a lower ramus especially below the premolars and the height is just 27.8 mm anterior to p2; the ramus under the cheek teeth protrudes lingually and the width at m1/m2 is 24.1 mm, comparatively broader than that of V 9959 (19.5 mm) which has a smaller height/width ratio (35.1%) than NWU V 1542 (52%). Consequently, NWU V 1542 develops a lower but slightly more robust mandible than V 9959. Furthermore, V 9959 has a longer diastema, estimated at 54 mm, and there is clearly a great decrease in the height of the horizontal ramus at the level of the diastema, forming a remarkable intersection angle between the slanted diastema and alveolar plane (about 22.4°). NWU V 1542, however, has a shorter diastema (38 mm), and the upper border of the mandible in front of p2 is just slightly lower than the alveolar plane without an obvious descent. Even though both mandibular symphyses end anterior to p2, V 9959 has a longer distance (30 mm) to p2 than that of NWU V 1542 (10.2 mm). In addition, V 9959 has two mental foramina and the larger one is beneath the i3 (“lower canine” in Qiu et al. 1998), the smaller one below the middle of the diastema, whereas only one mental foramen is visible on the NWU V 1542. However, the location and number of mental foramina can’t be regarded as a stable feature at the specific level for it often varies within species, as in M. elatus, which develops different numbers of mental foramina. It should be noted that the size differences of NWU V 1542 and V 9959 is quite minimal compared with those between NWU V 1542 and B. betpakdalensis and does not exceed the range of intraspecific variation. The differences of mandibular morphology, particularly the front end of the ramus between NWU V 1542 and V 9959, may be the result of sexual dimorphism.
Both NWU V 1542 and V 9959 have been discovered from the same white sandstone at the bottom of the Middle Member of Xianshuihe Formation and both fossil localities—Taowan and Huangyangtou—are located at the west flank of the syncline in the Lanzhou Basin, less than 4 km away from each other. Just as discussed above, V 9959 shows many similarities with B. betpakdalensis regarding mandibular morphology except the marked decline of the ramus height at the diastema, and the smaller size. Therefore, the two mandibles from the early Miocene Lanzhou Basin probably represent the same species of Borissiakia. Here we use Borissiakia huangheensis as the specific name for the smaller-sized species of Borissiakia.
Comparison with Metaschizotherium wetzleri
Metaschizotherium wetzleri has only been found in the latest Oligocene and early Miocene (MN2a and MN3) of Europe, particularly in the Lower Freshwater Molasse of South Germany and the North Bohemian graben in the Czech Republic. Because of the scarcity of fossils, its phylogenetic relationship is still uncertain, and the species had been assigned to Chalicotherium wetzleri (Kowalewsky 1873), Schizotherium wetzleri (von Koenigswald 1932), Metaschizotherium cf. wetzleri (Fejfar et al. 1997), and Moropus wetzleri (Heissig and Fejfar 2013). Recently, Heissig and Fejfar (2013) studied the materials of Metaschizotherium cf. wetzleri from the early Miocene (MN3a) of Merkur-Nord in the Czech Republic (Fejfar et al. 1997) and assigned them to P. schlosseri. At present, it is clear that M. wetzleri represents a middle-sized schizotheriine, but its taxonomy requires further study (Coombs 2009; Heissig and Fejfar 2013).
For that reason, the comparisons with NWU V 1542 are rather limited. Both taxa develop slender mandibles with relatively straight lower borders, and the symphysis of M. wetzleri (BSPG 1867 XII 5) also ends anterior to p2. We roughly calculate the height of the ramus posterior to m3, between m3/m2 and between m1/p4, and the results are similar to those for NWU V 1542 (Fig. 7). A minor difference is that in M. wetzleri, the lower border of the ramus below m3 shows a weak concave curvature, which is different from the straight border of NWU V 1542. Besides, the lower molars of M. wetzleri are slightly larger but the m3 of NWU V 1542 seems broader than the former with a higher width/length ratio (Fig. 6). Coombs (2009) proposed that the trigonids and talonids of m2 and m3 of M. wetzleri are close to the same width, which is similar to m3 of NWU V 1542 but the talonid of m2 is obviously wider than the trigonid. In brief, since the generic status of M. wetzleri is unclear and the material is rather rare, it is difficult to compare more diagnostic characters of M. wetzleri with the Huangyangtou chalicothere.
Comparison with Moropus elatus
Moropus is the best-known genus of the Schizotheriinae and probably originated in Eurasia and then migrated to North America close to the Oligocene/Miocene boundary (Coombs 1973, 1978a, 1989; Coombs and Hunt 2015). In the early Miocene, the genus was widely distributed in the New World and even dispersed to Central America (Wood and Ridgwell 2015). Among the six species of the genus, M. elatus is one of the most important comparative species in the study of schizotheriines, based on rich materials (Coombs 1978a).
The similar features shared by M. elatus and NWU V 1542 mainly lie in the short symphysis, elongation of lower molars, straight lophids on the trigonid and talonid, separated metaconid and metastylid, and lack of a lingual cingulum on the lower molars. However, there exist remarkable differences between both taxa. M. elatus is the largest species of the genus and also larger than many other schizotheriine chalicotheres of the early-middle Miocene. Compared with NWU V 1542, M. elatus develops a higher and stouter mandibular ramus, with a longer diastema that extends to below the base of p2, larger lower molars with well-developed anterolabial and posterolabial cingula, and a much longer molar row (109 ~ 150 mm), indicating an increased body size (Fig. 6, 7 and Table 1).
Coombs et al. (2001) also described material of M. oregonensis from the upper John Day Formation, Oregon, which represents the earliest Moropus in North America. It is a small chalicothere with a less elongated molar row and is regarded as a primitive taxon. Although the known material of M. oregonensis are few upper teeth and postcranials, which cannot be directly compared with NWU V 1542, a right mandible of M. cf. oregonensis from the St. Marks River, Leon County, Florida can be well compared with the Lanzhou specimen. In M. cf. oregonensis, the diastema (20 mm), premolar row (38 mm) and the whole length of cheek teeth (103.9 mm) are much shorter than those in NWU V 1542; its lower molars are also smaller (Fig. 6), which suggest that M. cf. oregonensis represents another small-sized chalicothere different from B. huangheensis.
Height of the horizontal ramus of early Miocene Schizotheriinae: measurements of Moropus elatus (AMNH 14,427) calculated based on Fig. 3 from Coombs (1978a, b); Metaschizotherium wetzleri was calculated from specimen 1867 XII 5; the measurements of Moropus elatus (CM 1604), Phyllotillon schlosseri (ZD 79b), Borissiakia betpakdalensis and Borissiakia huangheensis (IVPP V 9959) are from Holland and Peterson (1914), Heissig and Fejfar (2013), Borissiak (1946), and Qiu et al. (1998), respectively
Discussion
Taxonomic status of the Lanzhou schizotheriine mandibles
The new chalicotheriid material from the early Miocene of China is described and compared with that of other Asian species, especially Borissiakia and Phyllotillon. Borissiakia has only been found in Kazakhstan previously and represents a comparatively large-sized schizotheriine chalicothere. Compared with other genera, NWU V 1542 shares more similarities with B. betpakdalensis in mandibular and dental morphology (Fig. 8). The most remarkable difference between the two groups is the size. Borissiak (1946) described two different-sized groups of B. betpakdalensis, probably caused by sexual dimorphism since this condition is common in chalicotheres (Coombs 1975). However, even the smaller individual of B. betpakdalensis is larger than NWU V 1542 and their size differences can hardly be explained by sexual but with interspecific variation. The other schizotheriine mandible from the early Miocene Lanzhou Basin (IVPP V 9959) was formerly referred to Phyllotillon, but just as discussed above, it resembles Borissiakia as well on the tapered anterior part of the ramus. The minor differences in size and morphology of NWU V 1542 and V 9959 can be explained by sexual dimorphism (Fig. 8). Therefore, we attribute the mandibles from the early Miocene Lanzhou Basin to Borissiakia huangheensis, but both represent different sexes.
Comparison of the mandibular morphology of Borissiakia: a Borissiakia huangheensis (IVPP V 9959) from Qiu et al. (1998), the angle measured by the authors; b Borissiakia huangheensis (NWU V 1542); c Borissiakia betpakdalensis from Borissiak (1946), mirrored for better comparison. Scale bar equals 5 cm
Another point that needs to be considered is the stratigraphy and location of the schizotheriine mandibles from Lanzhou. Huangyangtou and Taowan are just separated by a small gully (Limashagou); the white sandstone at the bottom of the Middle Member of the Xianshuihe Formation is widespread and serves as a good marker bed for stratigraphic correlation in the basin. Furthermore, there is no evidence to suggest that NWU V 1542 and IVPP V 9959 had different dietary preferences and could have inhabited different ecological niches within the same habitat. Considering competitive exclusion, there would not have been enough space to support different groups of schizotheriines and other large perissodactyls like the giant rhinos in such a limited basin. From this perspective, it is also reasonable to attribute the two mandibles to the same species, Borissiakia huangheensis, suggesting that the schizotheriine Borissiakia once migrated between Central Asia and Northwest China during the latest Oligocene and early Miocene.
Paleoenvironmental indicator
Mesowear and microwear analyses suggest schizotheriines were not strictly leaf browsers but consumed more or less abrasive and fibrous materials like bark, branch or twigs (Moropus, Tylocephalonys, Ancylotherium), or fruit with hard seeds (Metaschizotherium bavaricum, Metaschizotherium fraasi) (Coombs and Semprebon 2005; Schulz et al. 2007; Coombs 2009; Fahlke and Coombs 2009; Schulz and Fahlke 2009). Different dietary niches may reflect diverse habitats, such as a moist forested environment for M. bavaricum and a drier forested environment for M. fraasi; Moropus elatus and Tylocephalonyx skinneri may have preferred well-watered riparian environments in semiarid savannas with seasonal rainfall (Coombs 1978a, b, 1979, 2009; Fahlke and Coombs 2009; Semprebon et al. 2011). Compared to the subfamily Chalicotheriinae, Schizotheriinae was more flexible regarding habitat preference, with ecological environments ranging from humid and dense forests to seasonally arid and open woodlands or savannas. Although the dental mesowear or microwear signatures of Borissiakia are unknown, the occurrence of B. huangheensis in the Zhangjiaping fauna of the Lanzhou Basin could indicate woodland during that period. That is also supported by the presence of large perissodactyls like Turpanotherium and Aprotodon in the fauna (Qiu and Xie 1997; Li et al. 2021). Turpanotherium is a slightly smaller paracerathere, and its dental features as well as postcranial anatomy suggest this giant rhino may have used the anteriormost part of the snout to gather food (leaves, twigs, and even bark of trees) (Qiu and Wang 2007). Aprotodon may have lived in sparse riparian mosaics within the semi-arid or arid part of Northwest China (Deng 2013). The coexistence of large perissodactyls suggests that the climatic and environmental conditions of the Lanzhou Basin were semiarid open woodlands with low seasonality during the early Miocene. In addition, the pollen assemblage of the basin during the late Oligocene and the early Miocene was dominated by Quercoidites-Liquidambarpollenites-Chenopodipollis; Liquidambarpollenites appeared, and xylophyta increased greatly. Herbaceous plants are mainly represented by Chenopodipollis and Compositae, suggesting that the paleoclimate probably changed to more humid than during the late Oligocene (Song et al. 2008). Today, the area is dominated by semi-arid temperate continental climate and vegetational regionalization belongs to the desert steppe zone of the western Loess Plateau with drought-tolerant plants. The appearance of B. huangheensis and other large perissodactyls suggests that the paleoenvironment during the early Miocene was different from today in being slightly moister, and that woodlands were more widespread in the Lanzhou Basin.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
References
Bonis L de, Bouvrain G, Koufos G, Tassy P (1995) Un crâne de chalicothère (Mammalia, Perissodactyla) du Miocène supérieur de Macédoine (Grèce): remarques sur la phylogénie des Chalicotheriinae. Palaeovertebrata 24:135-176
Borissiak AA (1946) A new chalicothere from the Tertiary of Kazakhstan. Trudy Paleontol Inst Akad Nayk USSR 13:1-134
Butler PM (1965) Fossil mammals of Africa. No. 18: East African Miocene and Pleistocene chalicotheres. Bull Br Mus Nat Hist Geol 10:165-237
Chavasseau O, Chaimanee Y, Coster P, Emonet E-G, Soe AN, Kyaw AA, Maung A, Rugbumrung M, Shwe H, Jaeger JJ (2010) First record of a chalicothere from the Miocene of Myanmar. Acta Palaeontol Pol 55:13-22. https://doi.org/10.4202/app.2009.0033
Chen SK (2008) A review on Chinese Neogene chalicotheres. In: Dong W (ed) Proceedings of the Eleventh Annual Meeting of the Chinese Society of Vertebrate Paleontology. China Ocean Press, Beijing, pp 31–41
Chen SK, Deng T, He W, Chen SQ (2012) A new species of Chalicotheriinae (Perissodactyla, Mammal) from the late Miocene in the Linxia Basin of Gansu, China. Vertebr Palasiat 50:53-73
Colbert EH (1934) Chalicotheres from Mongolia and China in the American Museum. Bull Am Mus Nat Hist 67:353-387
Coombs MC (1973) The Schizotheriinae (Mammalia, Perissodactyla, Chalicotheriidae) with emphasis on the Genus Moropus. Dissertation, Columbia University, New York
Coombs MC (1974) Ein Vertreter von Moropus aus dem europäischen Aquitanien und eine Zusammenfassung der europäischen postoligozänen Schizotheriinae (Mammalia, Perissodactyla, Chalicotheriidae). Sitzber Österr Akad Wiss, Math-Nat Kl 182:273-288
Coombs MC (1975) Sexual dimorphism in chalicotheres (Mammalia, Perissodactyla). Syst Zool 24:55-62. https://doi.org/10.2307/2412697
Coombs MC (1978a) Reevaluation of early Miocene North American Moropus (Perissodactyla, Chalicotheriidae, Schizotheriinae). Bull Carnegie Mus Nat Hist 4:1-62
Coombs MC (1978b) Additional Schizotherium material from China, and a review of Schizotherium dentitions (Perissodactyla, Chalicotheriidae). Am Mus Novit 2647:1-18
Coombs MC (1979) Tylocephalonyx, a new genus of North American dome–skulled chalicotheres (Mammalia, Perissodactyla). Bull Am Mus Nat Hist 164:1-64
Coombs MC (1989) Interrelationships and diversity in the Chalicotheriidae. In: Prothero DR, Schoch RM (eds) The Evolution of Perissodactyla. Oxford University Press, New York, pp 438-457
Coombs MC (2004) Moropus merriami in the early Barstovian Lower Snake Creek fauna of Nebraska, with comments on biogeography of North American chalicotheres. Bull Am Mus Nat Hist 285:191-208. https://doi.org/10.1206/0003-0090(2004)285<0191:C>2.0.CO;2
Coombs MC (2009) The chalicothere Metaschizotherium bavaricum (Perissodactyla, Chalicotheriidae, Schizotheriinae) from the Miocene (MN5) Lagerstätte of Sandelzhausen (Germany): description, comparison, and paleoecological signifificance. Paläont Z 83:85-129. https://doi.org/10.1007/s12542-009-0004-x
Coombs MC, Cote SM (2010) Chalicotheriidae. In: Werdelin L, Sanders WJ (eds) Cenozoic Mammals of Africa. University of California Press, Berkeley, pp 659-667
Coombs MC, Hunt RM Jr (2015) New material of Moropus (Perissodactyla, Chalicotheriidae, Schizotheriinae) from the early Hemingfordian Rose Creek Member of the John Day Formation, Oregon, U.S.A. J Vertebr Paleontol 35:e1009992. https://doi.org/10.1080/02724634.2015.1009992
Coombs MC, Semprebon GM (2005) The diet of chalicotheres (Mammalia, Perissodactyla) as indicated by low magnification stereoscopic microwear analysis. Abstracts of Papers, 65th Annual Meeting, Society of Vertebrate Paleontology, Mesa, Arizona. J Vertebr Paleontol 25(3 Supp):47A
Coombs MC, Hunt RM Jr, Stepleton E, Albright LB III, Fremd T (2001) Stratigraphy, chronology, biogeography, and taxonomy of early Miocene small chalicotheres in North America. J Vertebr Paleontol 21:607-620. https://doi.org/10.1671/0272-4634(2001)021[0607:SCBATO]2.0.CO;2
Deng T (2013) Incisor fossils of Aprotodon (Perissodactyla, Rhinocerotidae) from the early Miocene Shangzhuang Formation of the Linxia Basin in Gansu, China. Vertebr Palasiat 51:131-140
Fahlke JM, Coombs MC (2009) Dentition and first postcranial description of Metaschizotherium fraasi Koenigswald, 1932 (Perissodactyla: Chalicotheriidae) and its occurrence on a karstic plateau–new insights into schizotheriine morphology, relationships, and ecology. Palaeontogr A 290:65-129. https://doi.org/10.1127/pala/290/2009/65
Fejfar O, Heizmann EPJ, Major P (1997) Metaschizotherium cf. wetzleri (Kowalewsky) from the early Miocene of Czech Republik and south Germany. In: Aguilar JP, Legendre S and Michaux J (eds) Actes du Congrès BioChroM’97. Mémoires et Travaux de l’Ecole Practique des Hautes Etudes, Institut de Montpellier, Montpellier, pp 707–709
Flerov KK (1938) Remains of Ungulata from Betpak–dala. Comptes Rendus Acad Sci 21: 94-96
Forster-Cooper C (1920) Chalicotheroidea from Baluchistan. Proc Zool Soc London 90:357-366. https://doi.org/10.1111/j.1469-7998.1920.tb07076.x
Gill T (1872) Arrangement of the families of mammals with analytical tables. Smithson Misc Collect 11:1-98.
Handa N, Kawabe S (2016) Femur of Schizotheriinae (Perissodactyla, Chalicotheriidae) from the lower Miocene Hiramaki Formation of the Mizunami Group in Gifu Prefecture, central Japan. J Vertebr Paleontol 36(4):e1131163. https://doi.org/10.1080/02724634.2016.1131163
Heissig K (1999) Family Chalicotheriidae. In: Rössner GE, Heissig K (eds) The Miocene Land Mammals of Europe. Verlag Dr. Friedrich Pfeil, Munich, Germany, pp 189-192
Heissig K, Fejfar O (2013) Die Säugetiere aus dem Untermiozän des Chomutov Beckens–I. Chalicotheriidae (Mammalia, Perissodactyla). Acta Mus Natl Prag B 69:7-64
Holland WJ, Peterson OA (1914) The osteology of the Chalicotheroidea with special reference to a mounted skeleton of Moropus elatus Marsh, now installed in the Carnegie Museum. Mem Carnegie Mus 3:189-406. https://doi.org/10.5962/p.211102
Kowalewsky W (1873) Monographie der Gattung Anthracotherium Cuv. und Versuch einer natürlichen Classification der fossilen Hufthiere. Palaeontogr 22:131-346
Li ZY, Li YX, Zhang YX, Xie K, Li ZC, Chen Y (2021) New material of Aprotodon lanzhouensis (Perissodactyla, Rhinocerotidae) from the Early Miocene in Northwest China. Geol J 56(9):4779-4787. https://doi.org/10.1002/gj.4212
Liu Y, Zhang ZQ (2012) New materials of Chalicotherium brevirostris (Perissodactyla, Chalicotheriidae) from the Tunggur Formation, Inner Mongolia. Geobios 45:369-376. https://doi.org/10.1016/j.geobios.2011.10.011
Owen R (1848) The Archetype and Homologies of the Vertebrate Skeleton. John Van Voorst, London
Pickford M (2020) Description catalogue of Chalicotheriidae (Mammalia, Perissodactyla) from the early Miocene of Napak, Uganda. Geo Pal Uganda 15:1-36
Qiu ZX, Wang BY (2007) Paracerathere Fossils of China. Science Press, Beijing
Qiu ZX, Xie JY (1997) A new species of Aprotodon (Perissodactyla, Rhinocerotidae) from Lanzhou Basin, Gansu, China. Vertebr Palasiat 35:250-267
Qiu ZX, Wang BY, Xie JY (1998) Mid–Tertiary chalicothere (Perissodactyla) fossils from Lanzhou, Gansu, China. Vertebr Palasiat 36:297-318
Qiu ZX, Wang BY, Qiu ZD, Xie GP, Xie JY, Wang XM (1997). Recent advances in study of the Xianshuihe Formation in Lanzhou Basin. In: Tong YS, Zhang YY, Wu WY, Li JL, Shi LQ (eds) Evidence for Evolution: Essays in Honor of Prof. Chung chien Young on the Hundred Anniversary of His Birth. China Ocean Press, Beijing, pp 177-192
Qiu ZX, Qiu ZD, Deng T, Li CK, Zhang ZQ, Wang BY, Wang XM (2013) Neogene Land Mammal Stages/Ages of China–Toward the Goal to Establish an Asian Land Mammal Stage/Age Scheme. In: Wang XM, Flynn LJ, Fortelius M (eds) Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology. Columbia University Press, New York, pp 29-83
Schulz E, Fahlke JM (2009) The diet of Metaschizotherium bavaricum (Chalicotheriidae, Mammalia) from the MN5 of Sandelzhausen (Germany) implied by the mesowear method. Paläont Z 83:175-181. https://doi.org/10.1007/s12542-009-0007-7
Schulz E, Fahlke JM, Merceron G, Kaiser TM (2007) Feeding ecology of the Chalicotheriidae (Mammalia, Perissodactyla, Ancylopoda). Results from dental micro- and mesowear analyses. Verh Naturwiss Ver Hamburg 43:5-31
Semprebon GM, Sise PJ, Coombs MC (2011) Potential bark and fruit browsing as revealed by stereomicrowear analysis of the peculiar clawed herbivores known as chalicotheres (Perissodactyla, Chalicotherioidea). J Mammal Evol 18:33-55. https://doi.org/10.1007/s10914-010-9149-3
Song ZC, Wang WM, Mao FY (2008) Palynological implications for relationship between aridification and monsoon climate in the Tertiary of NW China. Acta Palaeontol Sin 47:265-272
von Koenigswald GHR (1932) Die Tertiären Wirbeltiere des Steinheimer Beckens: Metaschizotherium fraasi n. g. n. sp., ein neuer Chalicotheriide aus dem Obermiocän von Steinheim a. Albuch–Bemerkungen zur Systematik der Chalicotheriiden. Palaeontogr 8:1–24
Wood AR, Ridgwell NM (2015) The first Central American chalicothere (Mammalia, Perissodactyla) and the paleobiogeographic implications for small-bodied schizotheriines. J Vertebr Paleontol 35: e923893. https://doi.org/10.1080/02724634.2014.923893
Yue LP, Heller F, Qiu ZX, Zhang L, Xie GP, Qiu ZD, Zhang YX (2000) Magnetostratigraphy and palaeo–environmental record of Tertiary deposits of Lanzhou Basin. Chin Sci Bull 45:1998-2002
Zapfe H (1979) Chalicotherium grande (Blainv.) aus der miozänen Spaltenfüllung von Neudorf an der March (Devinská Nová Ves), Tschechoslowakei. Neue Denkschr Naturhist Mus Wien 2:1-282
Zhai YP, Cai TL (1984) The Tertiary system of Gansu Province. Gansu Geol 2:1-40
Zhang P (2015) Magneto stratigraphy and paleoenvironmental evolution of the middle Eocene–early Miocene deposits in the Lanzhou Basin, northwest China. Dissertation, University of Chinese Academy of Sciences, Beijing
Acknowledgements
We are very grateful to Prof. Margery C. Coombs (University of Massachusetts, USA) for her great help, beneficial discussions, and comments on the material. We especially appreciate the important copy of her translation of Borissiak’s (1946) paper. She also provided other references and pictures of fossil materials for comparison, and improved the English language of the manuscript. We thank Dr. Shaokun Chen (Hebei GEO University) for his helpful discussions. We also thank Tanju Kaya for providing a reference. Thanks to Yuangang Yue (Northwest University) for helping take pictures of the material and Jie Sun (State Key Laboratory of Continental Dynamics, Northwest University) for scanning the specimen and reconstructing the 3D model. We thank the editor Darin Croft, associate editor Ornella Bertrand, and two anonymous reviewers for their comments and reviews which greatly improve the quality of this work.
Funding
Open access funding provided by Swedish Museum of Natural History. The present research was financially supported by the National Natural Science Foundation of China (NSFC 42172010). ZY L sincerely appreciates the China Scholarship Council (no. 202106970018) for one year of research as a visiting PhD student with TM at the Swedish Museum of Natural History.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. ZYL, YXZ, KX, and YXL participated in the fieldwork. ZYL, TM, and YXL participate in the discussion and analysis of the material. The first draft of the manuscript was written by ZYL and revised by TM. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Mörs, T., Zhang, Y. et al. New Material of Schizotheriine Chalicothere (Perissodactyla, Chalicotheriidae) from the Xianshuihe Formation (Early Miocene) of Lanzhou Basin, Northwest China. J Mammal Evol 29, 877–889 (2022). https://doi.org/10.1007/s10914-022-09619-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-022-09619-3