Abstract
How cancer patterns in humans compare to those of other species remains largely unknown and there is an even bigger knowledge gap for rare cancers like male breast cancer. One Health is a convergence of human and animal healthcare that encourages cross-pollination of medical research uniting human and veterinary medicine. Recognising that breast cancer occurs spontaneously in other male species (e.g. primates, canines, felines), and knowing that no laboratory models exist for male breast cancer, which limits our ability to perform functional studies, we explored the feasibility of applying One Health to breast cancer in men by conducting a narrative review of the topic. Spontaneous development of breast cancer was reported in captive male primates and in companion canines and felines. Some parallels in tumour biology of human male breast cancer with canines and primates were found. The age distribution, pattern of biomarker expression and metastasis were similar, with mammary tumours typically detected after two-thirds of average lifespan. However, instances of triple negative and inflammatory breast cancer, which are rarely observed in human male breast cancer, were found in canines and histological classification was inconsistent between species. These disparities need redressing to enable full exploration of the One Health paradigm in rare cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
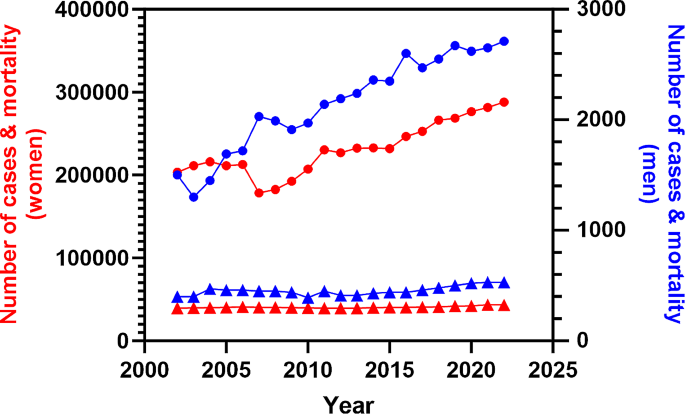
Some 400 men in the UK and an estimated 2800 in the US receive a breast cancer (BC) diagnosis each year, which is considerably fewer than the 56 000 and 297 000 women who are diagnosed, respectively, accounting for less than 1% of all breast cancer diagnoses worldwide [1]. However, as average lifespan increases, there has been a noticeable rise in male BC prevalence [2, 3], reflected in age-standardised data collected by the American Cancer Society over the last two decades [4, 5]. Indeed, a compilation of these data from 2002 to 2022 shows that the number of cases of male BC has increased from 1500 to 2710 per 100,000 persons (Fig. 1).
Incidence (circles) and mortality (triangles) trends of male (blue line, right axis)) and female (red line; left axis) breast cancer in the United States from 2002 to 2022. Graph constructed using data reported in the annual ‘Cancer Statistics’ publication by CA: A Cancer Journal for Clinicians [4, 5]
As BC in men is rare, it has been historically difficult to collect sufficient samples for studies to extend beyond anecdotal findings. This gap has been addressed through the establishment of the Male Breast Cancer Consortium and International Male Breast Cancer Program [6, 7], where large numbers of cases have been collected and centralised, allowing more rigorous characterisation. The focus of these consortia has been to collect formalin-fixed paraffin embedded tissues, predominantly. These have shown that male BC more often expresses estrogen (ER), progesterone (PR) and androgen receptors (AR), with a lower likelihood of human epidermal growth factor 2 (HER2) overexpression [6, 8,9,10]. Invasive ductal carcinoma accounts for approximately 83% of all male BC cases whereas lobular cancer is infrequent in men due to the absence of lobule development [11]. Other forms, including cribriform, intraductal papillary, mucinous, micropapillary, and papillary phenotypes, have also been reported in men, with papillary subtype showing a higher prevalence compared to women [8]. The principal molecular subtype in male BC is luminal A, with lower frequencies of luminal B and basal-like subtypes, including triple negative [6, 8].
Genomics and transcriptomics studies, indicate further differences between male and female BC, leading to the identification of two exclusive subgroups in men: luminal M1 and M2, distinguished by their unique tumour characteristics [12] and independent of the PAM50 subtypes in women. These findings, along with others, point towards male BC possessing a distinct genetic, histological, and receptor expression profile [6, 13,14,15].
While differences that separate male and female BC are being uncovered, the scarcity of male cases means that pre-clinical laboratory models are lacking. Numerous mouse models exist to study female BC [16], however male mice have only vestigial mammary tissue and lack nipples [17, 18], limiting their use as models. Recently, an ovine model has been proposed as a useful comparative model to understand the role of the microenvironment of the male mammary gland [19]. The “One Health” concept serves as a useful paradigm that leverages the shared knowledge of anatomy, pathology and physiology between human and veterinary medicine [20]. This approach highlights the potential of cross-species collaboration which has been demonstrated in various research studies [21, 22]. One Health was applied to investigate naturally-occurring osteoarthritis in canines to better understand the pathogenesis, molecular mechanisms, and potential treatment strategies relevant to humans [23]. Another study explored the similarities between humans and animals, particularly canines and equines, in tendon structure, function, and pathology [24] The One Health philosophy has also been proposed as a way of accelerating our understanding of understanding the pathology, diagnosis and treatment of mammary cancer across species [25, 26].
The translatability of spontaneously developed mammary cancer in non-human mammals, notably canines and felines is garnering interest [27,28,29,30] with previous research showing that spontaneous animal tumours are viable translational models in female BC [31, 32]. As companion animals often share the same environment as their owners and adopt some of their traits, they may develop the same comorbidities, including physical inactivity, a recognised risk factor for BC development [33, 34]. This has come on the back of detailed investigations into the genomic landscape of canine cancers which now exist alongside various analytical tools and images on public repositories [35]. However, there are no investigations into the applicability of using spontaneously developed mammary tumour in non-human male animals to model BC in men. Therefore, the aim of this work was to review the literature on spontaneously developed BC in non-human male mammals to determine its frequency and similarity to BC in male humans with a view to using these as pre-clinical models, which do not exist currently for male BC.
Literature Searching
For this narrative review, a comprehensive search of databases, Ovid MEDLINE® (1946 to June 12, 2023), Embase Classic + Embase (1947 to June 12, 2023), CAB Abstracts (1973 to June 12, 2023), All EBM reviews, Web of Science Core Collection, and SCOPUS, was conducted without restrictions on year or language. The search strategy involved compiling synonymous search terms for “breast,” “cancer,” and “men,” which were then combined with a comprehensive list of common animals from major mammalian classes.
Non-human Primates
Non-human primates share over 90% of DNA with humans. This genetic similarity offers comparability in research findings and explains why they are sometimes used in medical studies. Scientists made significant discoveries about diseases, disorders, prevention, and treatments for both humans and animals, tying in the ‘one approach’ technique long before One Health was touted and applying results from studies on non-human primates to humans. A prime example of this is the discovery of insulin over a century ago [36] which laid the foundation for this type of approach, demonstrating a compelling basis for developing novel treatment modalities and harnessing comparative research efforts across species. More recently, the relative value of using non-human primates to model BC has been reviewed [37].
Case reports of BC detected in a handful of male non-human primates in captivity during routine physical examinations have been described. Species include rhesus macaque (Macaca mulatta), squirrel monkey (Saimiri sciureus), and orangutan (Pongo pygmaeus) [38,39,40]. A mammary lesion was found in the rhesus macaque, diagnosed as a spontaneous ductal carcinoma in situ (DCIS), which is a pre-invasive lesion [38]. In women DCIS is picked up frequently as one of the unintended consequences of national breast screening programmes [41]. However, DCIS is rarely seen in men, who typically present with more advanced disease [42]. In the squirrel monkey, an elevated subcutaneous nodule was surgically removed by wide excisional biopsy and diagnosed as an adenocarcinoma. No further treatment was given, but when the monkey was euthanised 18 months later for a comorbidity, a positive lymph node was identified [40]. The tumour resected from the male orangutan was subjected to the same diagnostic work up as human BC and immunohistochemistry revealed the expression of hormone receptors. The animal received tamoxifen at the same dose as humans, but the disease progressed. Switching to anastrozole, an aromatase inhibitor, slowed progression and the animal died from unrelated causes 4.5 years after diagnosis [39]. This treatment plan mirrored that recommended by ASCO for management of human male BC [43]. In another primate, BC was detected as an incidental finding in an autopsy of a male Humboldt’s white-fronted capuchin (Cebus albifrons) who died after being attacked by a male baboon [44]. A hormone receptor positive grade 2 tubular carcinoma, which is rare in men [8] was diagnosed.
Non-primate Male Species
Forty papers reported mammary tumours in non-human non-primate male species, with a higher prevalence in canines and felines. A summary of species and age distribution is shown in Table 1. Mammary tumours typically occurred after two-thirds of the average lifespan. Canines comprised most cases, across 33 different breeds, with Cocker Spaniel (n = 14), German Shepherd (n = 6), and Dachshund (n = 6) most commonly reported, suggesting possible breed associations [45,46,47,48,49,50,51,52,53,54,55,56,57]. Indeed, previous studies have noted a preponderance of mammary tumours in these breeds among female canines [58]. Felines were the second most represented species, mainly in Domestic short-hairs, followed by four Siamese, three Domestic long-hair, and one Persian cat [59,60,61,62]. A single case of a simple ductal papilloma was reported in a captive maned wolf (Chrysocyon brachyurus) [63]. Papillary subtypes are seen more frequently in human male than in female BC [8]. Canines, felines and the lupine were diagnosed at an older age, median 10 years (range 1–15, canine) and median 11.5 years (range 3.5–19, feline).
Of the 84 reported cases of male mammary tumours in canines, 29 were malignant (Table S1). This classification was based on published guidelines for the histological classification for canine mammary tumours [81]. The remaining benign neoplasms included simple adenomas, fibroadenomas, intraductal papilloma, and benign mixed tumours. In felines, all mammary neoplasms identified were reported as malignant, except one case of cystic adenoma papilliferum. Most of the subtypes diagnosed were aggressive, with two distinct histological diagnoses observed: metastatic ductal adenocarcinoma and invasive micropapillary carcinoma [62, 71]. Thirty-nine cases reported in one paper were described as “mammary adenocarcinoma” without detailed histological classification [61]. However, mammary cancers in felines tend to lack heterogeneity and are usually more aggressive [82]. Indeed, felines had the highest proportion of tumour-related deaths among all species described in this review. Formal classifications for mammary tumours in domestic species, including canines and felines, do exist [83] and whilst adopted widely, this may not always be universal.
Biomarkers used to classify human BC have been described in non-primate male animals (Table 2).
While complete data for all cases was patchy, 19 cases across 9 papers documented the three key biomarkers used for reporting human BC (ER, PR, HER2), and in some cases, Ki-67. These cases were predominantly canine [45, 48, 53, 55, 57, 84,85,86], with one feline [62] and one lupine [63]. Triple-negative mammary cancer was observed in three male dogs, an unusual finding in human males, where BC is predominately hormone receptor positive [8]. However, ER-positivity was reflected in most of the cases where ER was examined. Despite this, endocrine therapy (tamoxifen) is not recommended in canines due to adverse side effects [87]. It is worth noting that in veterinary medicine and basic sciences, immunohistochemistry is not always conducted to the same rigorous standards required for clinical reporting in humans. This would need to be addressed were a One Health approach to be implemented.
Other Species
Among male rodents, eight out of ten reported BC cases were malignant tumours. Three were in rats (Rattus norvegicus); two in pets and one in a Wistar rat from a breeding colony, comprising two ductal mammary carcinoma and one papillary mammary carcinoma [72, 73]. A handful have been reported in male guinea pigs (Cavia porcellus) including invasive papillary carcinoma, solid simple mammary carcinoma, papillary cystadenocarcinoma, or benign tumours [74,75,76,77,78,79]. One case, a solid anaplastic adenocarcinoma, was reported in an intact male pet rabbit (Oryctolagus cuniculus) aged 7 years [80].
Although the focus of this paper was to explore the spontaneous development of male BC in non-human males, it is known that spontaneous tumour initiation can occur in oncogene-driven transgenic mice, typically MMTV-PyMT strains, which closely parallel human BC development and progression [88]. In a different transgenic strain FVB/N-Tg(MMTV-PyVT)634Mul/J (known as PyVT; [89]) mammary tumours developed spontaneously in male animals from 14 weeks of age which expressed ERα and -β, PR and HER2 [90]. HER2 is very rare in male BC [8], although ERβ has been reported [91]. Tumour burden was reduced following treatment with cisplatin but not paclitaxel or tamoxifen [90]. The latter finding is unusual as tamoxifen is typically first line choice for male BC [43]. Indeed, lack of response to tamoxifen in this transgenic mouse strain and its poor tolerance in canines [87] point to dissimilarities in physiology between species.
Management and Outcomes
Animals tended to be diagnosed with larger tumours compared to the average 2.4 cm tumour size in human male BC [92] as shown in Table S2. This discrepancy may arise as animals depend on their owners to notice the tumour, while in humans, palpable lumps would trigger suspicion. That said, BC in men is frequently diagnosed at a later stage due to a lack of awareness and stigma surrounding what is perceived by the stigma surrounding what is perceived by the public as a female cancer [93, 94]. Soft tissue metastases, but not the more common bone metastasis seen in male BC was observed in 8 animals. This aligns with previous studies highlighting a low incidence of bone metastasis in animals [95].
Surgery was performed in most canines (80/84), typically mastectomy and lumpectomy, like humans. Similarly, in felines all mammary tumours were managed surgically, with half of those unspecified. Lymph nodes were resected in two cases during radical mastectomy or lumpectomy [62, 71]. The specific surgical interventions for canine and felines were reported in Table 3.
Post-operative chemotherapy with doxorubicin and cyclophosphamide was administered in two feline cases [61], again similar to humans. In canines, two simple mammary carcinoma cases received Cytocristin chemotherapy post-lumpectomy while two inflammatory mammary carcinomas were managed with analgesics and anti-inflammatories [46, 85, 96]. The cases of inflammatory breast cancer (IBC) presented like humans, with rapid onset of skin erythema and tumour emboli in the dermal lymphatics however chemotherapy is preferred in humans due to its aggressive nature [98]. This was reflected in the short lifespan of these canines’ post-diagnosis. The wolf with a simple ductal mammary papilloma received unspecified surgery [63]. In rodents, one rat underwent a simple mastectomy, while two received a lumpectomy with cisplatin electrochemotherapy [72, 73]. Lumpectomy with regional node removal was performed in one guinea pig [79].The rabbit with solid anaplastic carcinoma underwent excisional biopsy [80]. However, treatment protocols cannot readily be compared in human and non-human species. The aim of the former is to prolong survival while the latter is to improve quality of live and alleviate symptoms.
In canines, only 5/49 cases reported metastasis [45, 51, 54, 84, 99]. Lymph node metastasis occurred in two cases, while metastasis to the contralateral mammary gland, lungs, and pelvic region each occurred once. A rabbit mammary tumour had metastasised to the lungs, pleural lining, and liver [80]. Metastasis was not documented among rats, guinea pigs or the wolf. The follow-up period and prognosis varied between 2 weeks to 77 months. Felines had the highest proportion of tumour-related deaths. The survival of male animals with mammary tumours across all species is summarised in Table 4.
Omics Studies
Genomic and transcriptomic data are being generated for canines and are starting to dissect the molecular pathways of BC in canines [28, 100]. Somatic and germline variants of BRCA1 and BRCA2 have been determined in felines [101]. Many similarities have been found, e.g. the frequency of somatic PIK3CA mutations, PI3K-Akt pathway deregulation, germline genetic variants in BRCA1/2 and p53-signalling; nevertheless, there is a notable lack of males in these studies.
Discussion
Male mammary tumours were predominantly reported in companion animals, likely due to the proximity to humans leading to a higher likelihood of incidental detection. Prevalence of mammary tumours in canines and felines may be attributed to their longer lifespan compared to rodents, increasing the probability of spontaneous oncogenic mutations. Tumour detection in all animals included in this study occurred past two-thirds of their lifespan, consistent with the older age distribution of BC in men [102].
Possible breed associations were identified with presentation in Spaniels, German Shephard, and Dachshunds. This is significant considering previous studies have noted a preponderance of mammary tumours in these breeds among female canines [58] and linked them to significant mutations found in human BC [103,104,105]. A study on Springer spaniels revealed a significant association between the development of mammary tumours and BRCA1 and BRCA2 polymorphisms, with 97% of diagnosed cases possessing these alleles [103]. In humans, BRCA mutations are present in 1–7% of the general population [106] and accounts for 5–10% of all breast cancer cases [106, 107]. However, BC in men is more typically associated with BRCA2 mutations which is linked to a higher lifetime risk of developing BC compared to BRCA1 carriers (1–5% versus 5–10%) [108].
BC classification in men is based on histological and molecular subtyping. Similar histological classifications exist between primates and humans and standardisation for canine and feline mammary tumour classification has been introduced [83]. Omics studies are still in their infancy for non-human species and males remain underrepresented. This is an area which deserves further study.
An unexpected finding was the identification of two less frequent manifestations of BC in humans, TNBC and IBC in male dogs. IBC closely mimicked the human disease in symptoms and histological features. Like male BC, IBC is also an uncommon form of BC accounting for just 2.5% of all cases in the US [109] with very few in men [110]. Both canine cases presented with painful erythematous mammary swellings alongside inflammatory infiltrate and dermal lymphatic vessels, like human cases [98, 109, 110]. Despite no reported metastasis, the short survival period in these canines mirrors the extremely poor prognosis observed in IBC in humans [98]. While 2 male canines diagnosed with TNBC had poor outcomes, dying within a few months of surgery or euthanised at time of diagnosis [45], a third remained recurrence-free for 6 months post-surgery [84].
While immunohistochemistry is used routinely in clinical workups for human BC for molecular classification and prognostication, this was less common in animal studies suggesting that veterinary medicine may prioritise tumour diagnosis and treatment over classification. Additionally, cost and availability of specialised testing in veterinary medicine, may further limit routine biomarker testing in animals. Indeed only 10% of the studies we examined reported on ER, PR, and HER2. This may change in the future, following a consensus statement from Brazil recommending a standard immunohistochemical panel for diagnosing mammary tumours in canines and felines, including ER, PR, Ki-67 and COX2 [111].
In contrast to BC management in men, where lumpectomy is uncommon due to limited breast tissue and adverse effects with adjuvant therapies, most animals in this study (where surgery was specified) underwent lumpectomies. An orangutan with a hormone-receptor positive tumour received hormonal adjuvant therapy at human dosages. Although its efficacy in animals remains unproven, the administration of anastrozole appeared to slow the lymph node enlargement, suggesting potential control of tumour growth. While the ATAC trial identified the benefits of anastrozole in the postmenopausal setting in women [112], its value in male BC has been insufficiently explored [113]. Nevertheless, the administration of adjuvant hormonal therapies in the orangutan demonstrates the potential for cross-species application of BC treatments.
The restrictive sample size across non-canine animals as well as insufficient and inconsistent reporting of the histology, biomarker expression, and prognosis of individual cases reduce the certainty of the conclusions made in comparison to human male BC. The relative recency of publication of the recommended guidelines for reporting of canine BC [111] limits the interpretation of reported histological features. Despite this, our work highlights pros, and cons of utilising animal models to understand human male BC. This needs redressing to enable full exploration of the One Health paradigm in rare cancers.
Data Availability
No datasets were generated or analysed during the current study.
Change history
23 April 2024
Author name has been corrected FROM First name: “Kirsty” Last name: “Luo-Yng Tay” TO First name: Kirsty Luo-Yng Last name: Tay. The correct reference should be “Tay et al” instead of “Luo-Yng Tay et al”.
References
Fox S, Speirs V, Shaaban AM. Male breast cancer: an update. Virchows Arch. 2022;480(1):85–93. https://doi.org/10.1007/s00428-021-03190-7. Epub 2021/08/31.
Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115(2):429 – 30. Epub 2008/05/15. https://doi.org/10.1007/s10549-008-0053-y. PubMed PMID: 18478326.
Reddington R, Galer M, Hagedorn A, Liu P, Barrack S, Husain E, et al. Incidence of male breast cancer in Scotland over a twenty-five-year period (1992–2017). Eur J Surg Oncology: J Eur Soc Surg Oncol Br Association Surg Oncol. 2020;46(8):1546–50. https://doi.org/10.1016/j.ejso.2020.01.009. Epub 2020/01/21.
Jemal A, Thomas A, Murray T, Thun M, Cancer statistics. 2002. CA Cancer J Clin. 2002;52(1):23–47. Epub 2002/01/30. https://doi.org/10.3322/canjclin.52.1.23. PubMed PMID: 11814064.
Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics. 2022. CA Cancer J Clin. 2022;72(1):7–33. Epub 2022/01/13. doi: 10.3322/caac.21708. PubMed PMID: 35020204.
Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International male breast Cancer Program. Annals Oncology: Official J Eur Soc Med Oncol. 2018;29(2):405–17. https://doi.org/10.1093/annonc/mdx651. Epub 2017/11/02.
Speirs V, Pollock S, Shaaban AM, Hanby AM. Problems (and solutions) in the study of male breast cancer. Rare Tumors. 2010;2(2):e28. https://doi.org/10.4081/rt.2010.e28. Epub 2010/12/09.
Humphries MP, Sundara Rajan S, Honarpisheh H, Cserni G, Dent J, Fulford L, et al. Characterisation of male breast cancer: a descriptive biomarker study from a large patient series. Sci Rep. 2017;7:45293. https://doi.org/10.1038/srep45293. Epub 2017/03/30.
Ottini L, Capalbo C, Rizzolo P, Silvestri V, Bronte G, Rizzo S, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45–58. https://doi.org/10.2147/bctt.S6519. Epub 2010/01/01.
Zelli V, Silvestri V, Valentini V, Bucalo A, Rizzolo P, Zanna I, et al. Transcriptome of male breast Cancer matched with germline profiling reveals Novel Molecular subtypes with possible clinical relevance. Cancers. 2021;13(18). https://doi.org/10.3390/cancers13184515. Epub 2021/09/29.
Senger JL, Adams SJ, Kanthan R. Invasive lobular carcinoma of the male breast - a systematic review with an illustrative case study. Breast Cancer (Dove Med Press). 2017. https://doi.org/10.2147/bctt.S126341. 9:337 – 45. Epub 2017/05/30.
Johansson I, Nilsson C, Berglund P, Lauss M, Ringner M, Olsson H, et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res. 2012;14(1):R31. https://doi.org/10.1186/bcr3116. Epub 2012/02/16.
Humphries MP, Sundara Rajan S, Droop A, Suleman CAB, Carbone C, Nilsson C, et al. A case-matched gender comparison transcriptomic screen identifies eIF4E and eIF5 as potential prognostic markers in male breast Cancer. Clin Cancer Res. 2017;23(10):2575–83. https://doi.org/10.1158/1078-0432.Ccr-16-1952. Epub 2016/12/18.
Moelans CB, de Ligt J, van der Groep P, Prins P, Besselink NJM, Hoogstraat M, et al. The molecular genetic make-up of male breast cancer. Endocr Relat Cancer. 2019;26(10):779–94. https://doi.org/10.1530/erc-19-0278. Epub 2019/07/25.
Piscuoglio S, Ng CK, Murray MP, Guerini-Rocco E, Martelotto LG, Geyer FC, et al. The genomic Landscape of male breast cancers. Clin Cancer Res. 2016;22(16):4045–56. https://doi.org/10.1158/1078-0432.Ccr-15-2840. Epub 2016/03/11.
Park MK, Lee CH, Lee H. Mouse models of breast cancer in preclinical research. Lab Anim Res. 2018;34(4):160–5. https://doi.org/10.5625/lar.2018.34.4.160. Epub 2019/01/24.
Szabo GK, Vandenberg LN. REPRODUCTIVE TOXICOLOGY: the male mammary gland: a novel target of endocrine-disrupting chemicals. Reproduction. 2021;162(5):F79–89. https://doi.org/10.1530/rep-20-0615. Epub 2021/01/13.
Stewart TA, Hughes K, Hume DA, Davis FM. Developmental stage-specific distribution of macrophages in Mouse Mammary Gland. Front Cell Dev Biol. 2019;7:250. https://doi.org/10.3389/fcell.2019.00250. Epub 2019/11/12.
Benjamin PD, Rachael CC, Anna LKC, Katie D, Andre Figueiredo B, Sonja J et al. An ovine model for investigation of the microenvironment of the male mammary gland. bioRxiv. 2023:2023.12.17.572059. https://doi.org/10.1101/2023.12.17.572059.
King TA. The One Medicine concept: its emergence from history as a systematic approach to re-integrate human and veterinary medicine. Emerg Top Life Sci. 2021;5(5):643–54. https://doi.org/10.1042/etls20200353. Epub 2021/08/07.
Gyles C, One Medicine O, Health, One World, Can Vet J. 2016;57(4):345–6. Epub 2016/04/05. PubMed PMID: 27041751; PubMed Central PMCID: PMCPMC4790223.
Partridge B, Rossmeisl JH. Jr. Companion animal models of neurological disease. J Neurosci Methods. 2020;331:108484. https://doi.org/10.1016/j.jneumeth.2019.108484. Epub 2019/11/17.
Smith RKW, McIlwraith CW. One health in tendinopathy research: current concepts. J Orthop Res. 2021;39(8):1596–602. https://doi.org/10.1002/jor.25035. Epub 2021/03/14.
Dan MJ, Crowley J, Broe D, Cross M, Tan C, Walsh WR. Patella tendinopathy zoobiquity - what can we learn from dogs? Knee. 2019;26(1):115–23. PubMed PMID: 30554911.
Vazquez E, Lipovka Y, Cervantes-Arias A, Garibay-Escobar A, Haby MM, Queiroga FL, et al. Canine mammary Cancer: state of the art and future perspectives. Animals. 2023;13(19):3147. https://doi.org/10.3390/ani13193147. PubMed PMID:.
Raposo TP, Arias-Pulido H, Chaher N, Fiering SN, Argyle DJ, Prada J, et al. Comparative aspects of canine and human inflammatory breast cancer. Semin Oncol. 2017;44(4):288–300. https://doi.org/10.1053/j.seminoncol.2017.10.012. Epub 2018/03/13.
Watson J, Wang T, Ho KL, Feng Y, Mahawan T, Dobbin KK, et al. Human basal-like breast cancer is represented by one of the two mammary tumor subtypes in dogs. Breast Cancer Res. 2023;25(1):114. https://doi.org/10.1186/s13058-023-01705-5. Epub 2023/10/04.
Bergholtz H, Lien T, Lingaas F, Sørlie T. Comparative analysis of the molecular subtype landscape in canine and human mammary gland tumors. J Mammary Gland Biol Neoplasia. 2022;27(2):171–83. https://doi.org/10.1007/s10911-022-09523-9. Epub 2022/08/07.
Liu D, Xiong H, Ellis AE, Northrup NC, Rodriguez CO Jr., O’Regan RM, et al. Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer. Cancer Res. 2014;74(18):5045–56. https://doi.org/10.1158/0008-5472.Can-14-0392. Epub 2014/08/02.
Zappulli V, De Zan G, Cardazzo B, Bargelloni L, Castagnaro M. Feline mammary tumours in comparative oncology. J Dairy Res. 2005;72. https://doi.org/10.1017/s0022029905001263. Spec 98–106. Epub 2005/09/27.
Zeng L, Li W, Chen CS. Breast cancer animal models and applications. Zool Res. 2020;41(5):477–94. https://doi.org/10.24272/j.issn.2095-8137. Epub 2020/07/07.
Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15(6):8195–205. Epub 2018/06/22. doi: 10.3892/ol.2018.8411. PubMed PMID: 29928319; PubMed Central PMCID: PMCPMC6004712.
Hughes K. Comparative mammary gland postnatal development and tumourigenesis in the sheep, cow, cat and rabbit: exploring the menagerie. Semin Cell Dev Biol. 2021;114:186–95. https://doi.org/10.1016/j.semcdb.2020.09.010. Epub 2020/10/22.
Dixon-Suen SC, Lewis SJ, Martin RM, English DR, Boyle T, Giles GG, et al. Physical activity, sedentary time and breast cancer risk: a mendelian randomisation study. Br J Sports Med. 2022;56(20):1157–70. https://doi.org/10.1136/bjsports-2021-105132. Epub 2022/11/04.
London CA, Gardner H, Zhao S, Knapp DW, Utturkar SM, Duval DL et al. Leading the pack: best practices in comparative canine cancer genomics to inform human oncology. Vet Comp Oncol. 2023;n/a(n/a). https://doi.org/10.1111/vco.12935.
Vecchio I, Tornali C, Bragazzi NL, Martini M. The Discovery of insulin: an important milestone in the history of Medicine. Front Endocrinol (Lausanne). 2018. https://doi.org/10.3389/fendo.2018.00613. 9:613. Epub 2018/11/09.
Dewi FN, Cline JM. Nonhuman primate model in mammary gland biology and neoplasia research. Lab Anim Res. 2021;37(1):3. https://doi.org/10.1186/s42826-020-00053-1. Epub 2021/01/06.
Roberts BM, Chumpolkulwong K, Tayamun S, Inamnuay L, Rungsipipat A, Lombardini ED. Mammary carcinoma in a male rhesus macaque (Macaca mulatta): histopathology and immunohistochemistry of ductal carcinoma in situ. J Med Primatol. 2014;43(3):213–6. https://doi.org/10.1111/jmp.12110. Epub 2014/03/22.
Carpenter NA, Crook EK. Mammary gland adenocarcinoma in a male Bornean Orangutan (Pongo Pygmaeus. J Zoo Wildl Med. 2017;48(1):224–7. https://doi.org/10.1638/2015-0303.1. Epub 2017/04/01.
Waggie KS, Tolwani RJ, Lyons DM. Mammary adenocarcinoma in a male squirrel monkey (Saimiri sciureus). Vet Pathol. 2000;37(5):505–7. https://doi.org/10.1354/vp.37-5-505. Epub 2000/10/31.
Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. European journal of cancer (Oxford, England: 1990). 2015;51(16):2296 – 303. Epub 2015/08/25. doi: 10.1016/j.ejca.2015.07.017. PubMed PMID: 26296293.
Wang F, Shu X, Meszoely I, Pal T, Mayer IA, Yu Z et al. Overall Mortality After Diagnosis of Breast Cancer in Men vs Women. JAMA oncology. 2019;5(11):1589-96. Epub 2019/09/20. doi: 10.1001/jamaoncol.2019.2803. PubMed PMID: 31536134; PubMed Central PMCID: PMCPMC6753503 fees from Novartis and Genentech; grants from Pfizer; and personal fees from Eli Lilly and Co, GlaxoSmithKline, Immunomedics, Macrogenics, and Seattle Genetics outside the submitted work. No other disclosures were reported.
Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, et al. Management of male breast Cancer: ASCO Guideline. J Clin Oncology: Official J Am Soc Clin Oncol. 2020;38(16):1849–63. https://doi.org/10.1200/jco.19.03120. Epub 2020/02/15.
Carvalho TP, Oliveira AR, Santos DOD, Cassali GD, Garcia APV, Pereira F, et al. Histopathologic and immunophenotypic profile of a spontaneous mammary carcinoma in a male Humboldt’s white-fronted capuchin (Cebus albifrons). J Med Primatol. 2022;51(1):49–52. https://doi.org/10.1111/jmp.12553. Epub 2021/11/14.
Machado MCA, Ocarino NM, Serakides R, Moroz LR, Sementilli A, Damasceno KA, et al. Triple-negative mammary carcinoma in two male dogs. J Vet Diagn Invest. 2020;32(1):94–8. Epub 2020/01/12. doi: 10.1177/1040638719898686. PubMed PMID: 31924140; PubMed Central PMCID: PMCPMC7003223.
Silva DMD, Kluthcovsky LC, Jensen de Morais H, Pallú GM, Dos Santos GC, Costa Castro JL, et al. Inflammatory mammary carcinoma in a male dog-case report. Top Companion Anim Med. 2019;37:100357. https://doi.org/10.1016/j.tcam.2019.100357. Epub 2019/12/16.
Park J-H, Noh D-J, Lee S-W, Jung D-U, Park J-K, Lee K-J. Diagnosis of Mammary Carcinoma in a castrated male Maltese dog. J Vet Clin. 2019;36(5):278–81. https://doi.org/10.17555/jvc.2019.10.36.5.278. Epub 2019/10/31.
Arias J, Paredes E, Torres C. Mammary carcinoma in a male dog: clinical and immunohistochemical characterisation. Arch De Med Vet. 2015;47:111–5.
Maiti S, Kumar D, Kumar S, Ar N, Mathew D, Palakkara S, et al. Mammary gland tumours in male dogs: a hormonal and tumour marker study. Veterinarski Arhiv. 2014;84:537–48.
Jabara AG. Two cases of mammary neoplasms arising in male dogs. Aust Vet J. 1969;45(10):476–80. https://doi.org/10.1111/j.1751-0813.1969.tb06594.x.
Zuchi TLVL, Spricigo JB, Lopatini CL, Faria JLM, Mueller EN, Sueiro FAR, et al. Mammary gland carcinoma in situ in a male dog: case report. Comp Clin Pathol. 2018;27(4):1097–101. https://doi.org/10.1007/s00580-018-2733-2.
Han J-H, Kim K-S, Kim J-H. Mammary gland tumors in three male dogs. Korean J Vet Res. 2016;56(4):229–32. https://doi.org/10.14405/kjvr.2016.56.4.229.
Saba CF, Rogers KS, Newman SJ, Mauldin GE, Vail DM. Mammary gland tumors in male dogs. J Vet Intern Med. 2007;21(5):1056–9. https://doi.org/10.1111/j.1939-1676.2007.tb03064.x.
Bearss JJ, Schulman FY, Carter D. Histologic, immunohistochemical, and clinical features of 27 mammary tumors in 18 male dogs. Vet Pathol. 2012;49(4):602–7. Epub 2011/03/29. doi: 10.1177/0300985811402843. PubMed PMID: 21441113.
Kwon SC, Yoo DY, Ko M, Lee KY, Kwak HH, Park IC, et al. Mammary gland tumors in a male Cocker Spaniel. Acta Vet Scand. 2017;59(1):20. https://doi.org/10.1186/s13028-017-0290-3. Epub 2017/04/13.
S PV, kumar KPS, Srilatha RVSND. Mammary neoplasm in a male dog - A Case Report. J Adv Veterinary Res. 2012;2(3):211–2.
Mamom P, Thengchaisri N, Sastravaha A, Ruangvejvorachai P, Mimapan S, Kaewmokul S, et al. Progesterone receptor expression in mammary gland tumor of male dogs. Kasetsart Veterinarians. 2012;22(2):14–22.
Edmunds G, Beck S, Kale KU, Spasic I, O’Neill D, Brodbelt D, et al. Associations between Dog Breed and Clinical features of mammary epithelial neoplasia in Bitches: an epidemiological study of submissions to a single Diagnostic Pathology Centre between 2008–2021. J Mammary Gland Biol Neoplasia. 2023;28(1):6. https://doi.org/10.1007/s10911-023-09531-3. Epub 2023/03/25.
Loukopoulos P, Sutton RH, Lynch P, Gee DC. Ectopic mammary carcinoma in a male cat. Vet Rec. 2007;160(6):203-4. Epub 2007/02/13. https://doi.org/10.1136/vr.160.6.203-a. PubMed PMID: 17293585.
Bastan A, Anadol E, Ozenc E, Yardimci B. A case of mammary neoplasm in a male cat. Veteriner Fakültesi Dergisi. 2007;54:139–40.
Skorupski KA, Overley B, Shofer FS, Goldschmidt MH, Miller CA, Sørenmo KU. Clinical characteristics of mammary carcinoma in male cats. J Vet Intern Med. 2005;19(1):52–5. https://doi.org/10.1892/0891-6640(2005)19<52:ccomci>2.0. Epub 2005/02/18.
Gregório H, Pires I, Seixas F, Queiroga F. Mammary invasive micropapillary carcinoma in a male cat: immunohistochemical description and clinical follow-up. Acta Veterinaria Hungarica. 2012;60(2):257–61. https://doi.org/10.1556/avet.2012.022.
Cassali GD, Bertagnolli AC, Ferreira E, Malta MCC. A simple ductal mammary papilloma in a male maned Wolf (Chrysocyon Brachyurus). J Vet Diagn Invest. 2009;21(1):153–5. https://doi.org/10.1177/104063870902100127.
Schoenbauer M. Mammary carcinoma of a male dog forming metastases [mammary tumour]. Revue de médecine vétérinaire 1982;133.
Aslan V, Çeşme H, Mutlu A, Cangül I, Salci H. Erkek Bir Köpekte Meme Bezinde Anaplastik Karsinoma Olgusu. J Uludağ Univ Fac Veterinary Med. 2017;36:25–8.
Damodaran S, Parthasarathy KR. Mammary neoplasms in male dogs. Indian J Veterinary Pathol. 1976;1(1):21–2.
Kumar P, Prasad V, Sreenu M, Chowdhary C. Mammary gland duct carcinoma in a male dog. Indian Veterinary J. 2018;95:89–90.
Pandey S, Bhargava M, Chandrapuria V, Das L, Kolte G. Mixed mammary tumour in a male dog. Indian J Veterinary Surg. 1983;4(1):70–2.
Salem H. Rzadki przypadek gruczolako-raka sutka (Adenocarcinoma metaplasticum) mammae u psa samca. Medycyna Weterynaryjna. 1973;29(5):306–7.
Oberoi M, Singh N, Kwatra M. Adenocarcinoma of mammary gland in a male dog. Agricultural Sci Digest. 1981;1(2):121–2.
Connah JG. Metastatic mammary adenocarcinoma initially misdiagnosed as a mast cell tumour in a male cat. Australian Veterinary Practitioner. 2016;46(2):56–9.
Lanza A, Pettorali M, Baldi A, Spugnini EP. Surgery and electrochemotherapy treatment of incompletely excised mammary carcinoma in two male pet rats (Rattus norvegicus). J Vet Med Sci. 2017;79(3):623–5. https://doi.org/10.1292/jvms.16-0578. Epub 2017/02/22.
Kalaiselvan P, Vijayakumar S, Hemalatha K, Murkunde YV, Herbert RA, Wells MY. Mass in the lateral cervical-thoracic region in a male Wistar rat. Adenocarcinoma of the mammary gland. Lab Anim (NY). 2009;38(9):288–91. https://doi.org/10.1038/laban0909-288. Epub 2009/08/25.
Grandi F, Monteiro L, Marietto-Gonçalves G, Rocha N. Mammary benign neoplasm diagnosed by fine needle aspiration biopsy in a guinea pig (Cavia porcellus). Acta Vet Brasílica. 2011;5(5):203–6.
Andrews E. Mammary neoplasia in the guinea pig (Cavia porcellus). Veterinarian. 1976;66(1):82–96.
Suárez-Bonnet A, Martín de las Mulas J, Millán MY, Herráez P, Rodríguez F. Espinosa De Los monteros A. Morphological and immunohistochemical characterization of spontaneous mammary gland tumors in the Guinea Pig (Cavia porcellus). Vet Pathol. 2010;47(2):298–305. PubMed PMID: 20106793.
Vienet V. New Pet animals. Mammary tumour in a male guinea pig. Point vétérinaire. 2002;33(229):1–4.
Amâncio B, Cangussú R, Pincinato S, Krause P. Achados citológicos em neoplasia mamária em porquinho da índia (Cavia porcellus) macho: Relato De Caso. Pubvet. 2021;15(6):1–3.
Walzl H, Leskova R. Spontanes, rezidivierendes Mammakarzinom Bei Einem Mannlichen Meerschweinchen. Wiener Tierärztliche Monatsschrift. 1979;66(5):186–7.
Summa NM, Eshar D, Snyman HN, Lillie BN. Metastatic anaplastic adenocarcinoma suspected to be of mammary origin in an intact male rabbit (Oryctolagus cuniculus). Can Vet J. 2014;55(5):475–9. Epub 2014/05/03. PubMed PMID: 24790235; PubMed Central PMCID: PMCPMC3992310.
Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48(1):117–31. Epub 2011/01/27. doi: 10.1177/0300985810393258. PubMed PMID: 21266722.
Hayes AA, Mooney S. Feline mammary tumors. Veterinary Clinics of North America -Small Animal Practice. 1985;15(3):513 – 20. https://doi.org/10.1016/S0195-5616(85)50054-6. PubMed PMID: WOS:A1985AJE9600005.
Zappulli V, Pena L, Rasotto R, Goldschmidt M, Gama A, Scruggs J, et al. In: Kiupel M, editor. Surgical Pathology of Tumors of Domestic Animals, mammary tumors. Volume 2: mammary tumors. Davis-Thompson Foundation; 2019.
Gopal K, Srinivasan P, Thangathurai R, Rajeswar J, Dharmaceelan S. Pathology of triple negative basal like mammary tumour in a male non-descript dog - case report. Indian J Veterinary Pathol. 2022;46:73–7.
Saranya R, Nagarajan K, Hemalatha S, Gokulakrishnan M, Balagangatharathilagar M, Rao G. Pathological studies on inflammatory mammary cystic papillary adenocarcinoma in a male dog. Indian J Veterinary Pathol. 2022;46(3):220–3.
Thakur M, Ramandeep RN, Gupta S, Gupta V. Cytological and histopathological characterization of squamous cell carcinoma from mammary region in a male dog: a case study. Indian J Veterinary Pathol. 2021;45(4):321–4.
Tavares WL, Lavalle GE, Figueiredo MS, Souza AG, Bertagnolli AC, Viana FA, et al. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet Scand. 2010;52(1):67. https://doi.org/10.1186/1751-0147-52-67. Epub 2010/12/24.
Attalla S, Taifour T, Bui T, Muller W. Insights from transgenic mouse models of PyMT-induced breast cancer: recapitulating human breast cancer progression in vivo. Oncogene. 2021;40(3):475–91. https://doi.org/10.1038/s41388-020-01560-0. Epub 2020/11/26.
Shishido S, Delahaye Ald, Beck A, Nguyen TA. The MMTV-PyVT transgenic mouse as a Multistage Model for Mammary Carcinoma and the efficacy of Antineoplastic Treatment. J Cancer Therapy. 2013;04:07:11. https://doi.org/10.4236/jct.2013.47138.
Shishido SN, Faulkner EB, Beck A, Nguyen TA. The effect of antineoplastic drugs in a male spontaneous mammary tumor model. PLoS ONE. 2014;8(6):e64866. https://doi.org/10.1371/journal.pone.0064866. Epub 2013/06/12.
Murphy C, Carder P, Lansdown M, Speirs V. Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol (EJSO). 2006;32(1):44–7.
Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2010;28(2):232–9. https://doi.org/10.1200/jco.2009.23.8162. Epub 2009/12/10.
Speirs V, Perspective. Not just for women. Nature. 2012;485(7400):S66. Epub 2012/06/01. https://doi.org/10.1038/485S66a. PubMed PMID: 22648505.
Co M, Lee A, Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9(10):3305–9. https://doi.org/10.1002/cam4.2953. Epub 2020/03/14.
Rosol TJ, Tannehill-Gregg SH, Corn S, Schneider A, McCauley LK. Animal models of bone metastasis. Cancer Treat Res. 2004;118:47–81. https://doi.org/10.1007/978-1-4419-9129-4_3. Epub 2004/03/27.
LM P, MR M. Surgical and chemotherapeutic management of mammary tumor in male dogs. J Indian Veterinary Association. 2011;9(3):55–6.
Schonbauer M. Mammatumoren Bei Ruden. Berliner Und Münchener. tierärztliche Wochenschrift. 1981;94(16):319–21.
Rea D, Francis A, Hanby AM, Speirs V, Rakha E, Shaaban A, et al. Inflammatory breast cancer: time to standardise diagnosis assessment and management, and for the joining of forces to facilitate effective research. Br J Cancer. 2015;112(9):1613–5. https://doi.org/10.1038/bjc.2015.115. Epub 2015/04/14.
Kwon JY, Moskwa N, Kang W, Fan TM, Lee C. Canine as a comparative and translational model for human mammary tumor. J Breast Cancer. 2023;26(1):1–13. https://doi.org/10.4048/jbc.2023.26.e4. Epub 2023/02/11.
Kim TM, Yang IS, Seung BJ, Lee S, Kim D, Ha YJ, et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat Commun. 2020;11(1):3616. https://doi.org/10.1038/s41467-020-17458-0. Epub 2020/07/19.
Govoni VM, Da Silva TC, Guerra JM, Pereira IVA, Queiroga FL, Cogliati B. Genetic variants of BRCA1 and BRCA2 genes in cats with mammary gland carcinoma. Vet Comp Oncol. 2021;19(2):404–8. https://doi.org/10.1111/vco.12685. Epub 2021/02/13.
Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26–36. https://doi.org/10.1002/cncr.32472. Epub 2019/10/08.
Rivera P, Melin M, Biagi T, Fall T, Häggström J, Lindblad-Toh K, et al. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69(22):8770–4. https://doi.org/10.1158/0008-5472.Can-09-1725. Epub 2009/11/06.
Thumser-Henner P, Nytko KJ, Rohrer Bley C. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Vet Res. 2020;16(1):30. https://doi.org/10.1186/s12917-020-2247-4. Epub 2020/02/02.
Arendt ML, Sakthikumar S, Melin M, Elvers I, Rivera P, Larsen M, et al. PIK3CA is recurrently mutated in canine mammary tumors, similarly to in human mammary neoplasia. Sci Rep. 2023;13(1):632. https://doi.org/10.1038/s41598-023-27664-7. Epub 2023/01/13.
Balmaña J, Díez O, Rubio IT, Cardoso F, Medical Oncology. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Annals of oncology: official journal of the European Society for. 2011;22 Suppl 6:vi31-4. Epub 2011/10/20. https://doi.org/10.1093/annonc/mdr373. PubMed PMID: 21908500.
Casaubon JT, Kashyap S, Regan JP. BRCA1 and BRCA2 mutations. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Sarang Kashyap declares no relevant financial relationships with ineligible companies. John-Paul Regan declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023. Disclosure: StatPearls Publishing LLC.; 2023.
Silvestri V, Leslie G, Barnes DR, Agnarsson BA, Aittomäki K, Alducci E, et al. Characterization of the Cancer Spectrum in Men with Germline BRCA1 and BRCA2 pathogenic variants: results from the Consortium of investigators of modifiers of BRCA1/2 (CIMBA). JAMA Oncol. 2020;6(8):1218–30. https://doi.org/10.1001/jamaoncol.2020.2134. Epub 2020/07/03.
Robertson FM, Cristofanilli M. A global approach to inflammatory breast cancer. Future oncology (London. England). 2011;7(1):25–30. https://doi.org/10.2217/fon.10.177. Epub 2010/12/23.
Tanhueco A, Youssef MMG. Inflammatory breast Cancer in men: a rare clinical case report and a literature review. Int J Surg Case Rep. 2021;80:105696. https://doi.org/10.1016/j.ijscr.2021.105696. Epub 2021/03/06.
Cassali G, Jark P, Gamba C, Damasceno K, Estrela-Lima A, Nardi A. Consensus regarding the diagnosis, prognosis and treatment of Canine and Feline Mammary tumors – 2019. Brazilian J Veterinary Pathol. 2020;13(3):555–74.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2. https://doi.org/10.1016/s0140-6736(04)17666-6. Epub 2005/01/11.
Giordano SH, Valero V, Buzdar AU, Hortobagyi GN. Efficacy of Anastrozole in male breast Cancer. Am J Clin Oncol. 2002;25(3).
Acknowledgements
We thank members of the Speirs group for useful discussion. SC was funded by an Elphinstone Scholarship from the University of Aberdeen.
Author information
Authors and Affiliations
Contributions
VS conceived the manuscript. KT and GC performed the literature search and data analysis. KT, GC, SC, GC and VS drafted the manuscript and prepared Figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors report there are no competing interests to declare. ChatGPT was used to translate papers that were screened in this study but published in languages other than English (references [65, 69, 78, 79 and 97]). No funding was received to assist with the preparation of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tay, KY., Cowan, G., Chatterji, S. et al. Exploring the One Health Paradigm in Male Breast Cancer. J Mammary Gland Biol Neoplasia 29, 8 (2024). https://doi.org/10.1007/s10911-024-09560-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10911-024-09560-6