Abstract
The human female breast gland is composed of branching epithelial ducts that extend from the nipple towards the terminal duct lobular units (TDLUs), which are the functional, milk-producing units of the gland and the site of origin of most breast cancers. The epithelium of ducts and TDLUs is composed of an inner layer of polarized luminal epithelial cells and an outer layer of contractile myoepithelial cells, separated from the vascular-rich stroma by a basement membrane. The luminal- and myoepithelial cells share an origin and in recent years, there has been increasing understanding of how these cell types interact and how they contribute to breast cancer. Accumulating evidence links stem/or progenitor cells in the mammary/breast gland to breast cancer. In that regard, much knowledge has been gained from studies in mice due to specific strains that have allowed for gene knock out/in studies and lineage tracing of cellular fates. However, there is a large histologic difference between the human female breast gland and the mouse mammary gland that necessitates that research needs to be done on human material where primary cultures are important due to their close relation to the tissue of origin. However, due to difficulties of long-term cultures and lack of access to material, human cell lines are of great importance to bridge the gap between studies on mouse mammary gland and human primary breast cells. In this review, we describe D492, a breast epithelial progenitor cell line that can generate both luminal- and myoepithelial cells in culture, and in 3D culture it forms branching ducts similar to TDLUs. We have applied D492 and its daughter cell lines to explore cellular and molecular mechanisms of branching morphogenesis and cellular plasticity including EMT and MET. In addition to discussing the application of D492 in studying normal morphogenesis, we will also discuss how this cell line has been used to study breast cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Histology of the Human Breast Gland
The human breast gland is an evolutionary modification of an apocrine sweat gland that originates from the epidermis and through interactions with the mesenchyme forms branching ducts that end in terminal duct lobular units (TDLUs) [1, 2] (Fig. 1). From birth to puberty, the breast glands are essentially the same in males and females [2]. At the onset of puberty in females, the breast gland develops further, grows into an increasingly elaborate ductoalveolar tree, embedded in collagen and adipose tissue. Periodic pulses in estrogen and progesterone production induce monthly cycles of branching morphogenesis of the glandular epithelium preparing for pregnancy and lactation. If pregnancy does not occur the epithelium partially involutes during menstruation preparing for a new round of branching morphogenesis. On the other hand, if pregnancy occurs the glandular epithelium progresses through to a more dramatic expansion, eventually reaching maximal differentiation during lactation [3]. These changes are driven by sustained hormonal production and by heterotypic interactions between the glandular epithelium and the surrounding vascular rich stroma [4]. The ducts and TDLUs are composed of an inner layer of polarized luminal epithelial cells and an outer layer of contractile myoepithelial cells that share a common origin [5,6,7] (Fig. 1). Myoepithelial cells have been shown to aid in polarization and maintenance of luminal epithelial differentiation in 3D culture [7,8,9]. Breast cancer rarely occurs in differentiated myoepithelial cells and their function has been associated with tumor suppression (reviewed in [10, 11]). During breast cancer initiation and progression, the histoarchitecture of the epithelium is disrupted. Initially, a localized lesion exhibiting an increase in proliferation and loss of polarization fills the ductal tree. Eventually, adhesion between cells is reduced, and in many cases the phenotype changes towards a more invasive, mesenchymal phenotype [12]. Some of these changes are accompanied by increased stemness and metastatic ability and development of drug resistance [12, 13].
Schematic figure of the human mammary gland and the spatial origin of the D492 cell line. Schematic view of the branching mammary gland and a schematic view of a terminal duct lobular unit (TDLU) which is composed of cluster of acini surrounded by cellular rich stroma. Also shown is a schematic view of an acinus (top), which is composed of a bilayer of epithelial cells, an inner layer of luminal epithelial cells (red) surrounded by an outer layer of myoepithelial cells (yellow). Suprabasal cells are depicted as blue cells. (Bottom). D492 was established from isolated suprabasal cells, 2D monolayer culture of D492 was stained with keratin 14 (myoepithelial cells) and keratin 19 (luminal epithelial cells). Cells were counterstained with DAPI. Bar = 50 μm. 3D culture showing GFP positive D492 cells forming structures reminiscent of TDLU. Bar = 50 μm
Differences Between the Human and Mouse Mammary Glands
In vivo models have contributed enormously to our knowledge of breast biology and cancer [3, 14, 15]. Knock-in and knock-out mutations have unraveled the functions of important tumor suppressors and or oncogenes and hundreds of mouse strains are available that capture phenotypic changes related to these gene interventions [14, 16]. While all this knowledge in terms of mouse mammary gland biology exist, it is important to acknowledge, that there are major differences between mouse and human mammary glands. This is due to histological differences between these two species. While both species’ mammary glands are embedded in adipose tissue, the mouse gland presents with very limited amount of collagenous stroma. The human female breast gland on the other hand is embedded in cellular rich intralobular stroma containing endothelial cells, fibroblasts and immune cells surrounded by interlobular collagen rich stroma [17]. Obviously, the species exhibit vast differences in life span (1 year compared to approx. 80 in humans), and therefore exhibit different sensitivity to cancerogenic insults [18, 19]. It is increasingly evident that breast cancer progression is an excellent example of Darwinian evolutionary principals, where cells that proliferate faster, survive better and adapt more proficiently to challenges in the environment. A one-year lifespan as is the case for the mouse is hardly a perfect model for recapitulating those parameters. Modeling human breast morphogenesis and cancer progression in vitro is therefore an important tool to explore molecular and cellular mechanisms that drive these phenotypic events. It is of great importance that we advance the understanding of how the human breast gland develops by improving cell culture models that recapture in vivo like morphogenesis. This will ultimately contribute to a better understanding of breast morphogenesis in normal conditions and in neoplasia.
Three-Dimensional Cell Culture to Explore Mammary Gland Morphogenesis and Cancer
There is growing interest in three-dimensional (3D) culture of human tissue due to the fact that conditions created in 3D more closely mimic in vivo conditions than culturing cells in conventional two dimensional (2D) monolayer culture where hiostoarchitecture is lost [20]. For decades, mammary epithelial cells have been cultured in 3D context and this work has been pioneered by several excellent scientists (reviewed in Simian and Bissell [21]). Emmerman and Pitelka demonstrated in 1977 that differentiated mammary cells could be cultured on floating collagen [22]. It was further shown that mammary epithelial cells were able to polarize and form milk protein when cultured on floating collagen [23, 24]. In addition, it has been shown in collagen culture albeit not floating gel that normal human breast myoepithelial cells regulate polarization of luminal epithelial cells, which is a function lacking in cancer-derived myoepithelial cells [8]. Recently, the group of Christina Scheel, succeeded in recapitulating branching morphogenesis in floating collagen gels using primary human breast epithelial cells [25, 26]. Another extracellular matrix that has been widely used to study breast/mammary gland morphogenesis and cancer is reconstituted basement membrane matrix (rBM) commonly referred to as Matrigel [27,28,29]. rBM has been successfully used to study polarization of normal breast epithelial cells and lack thereof in cancer cells [30, 31]. With improved cell culture methods such as better cell culture media, it is now possible to grow breast cancer-derived organoids for longer periods in 3D culture [32]. This is of great importance, since this enables drug screening using cancer-derived organoids, which forms a platform for future precision cancer medicine. In addition to collagen and rBM, researchers have begun testing new chemically defined matrices to grow breast epithelial cells. Recently, Sokol et al. described chemically defined hydrogel to cultivate breast epithelial cells. Under these cell culture conditions, primary breast epithelial cells self-organize into branching ducts with spatially correctly located luminal and basal cells [33]. Collectively, cell culture techniques have been improving through the years allowing breast epithelial cells to be cultivated in an environment that supports in vivo-like morphogenesis seen in normal and malignant mammary glands.
Cellular Origin of the D492 Cell Line
The search for the true human breast epithelial stem cells has been a long journey and a matter of debate within the research field for many years and still is. We have witnessed great success in cell lineage tracing and hunt for stem cells in the mouse mammary gland, where numbers of papers have demonstrated the presence of stem and progenitor cells [34,35,36]. For obvious reasons the linage tracing of breast epithelial stem/progenitor cells in vivo in humans is not possible. Instead, studies on human mammary gland rely on tissue from biopsies such as reduction mammoplasties and on established cell lines. Reduction mammoplasties have been a great resource for cell biologists studying breast development and breast cancer due to the large amount of material available from each biopsy and due to the normal histology of the tissue. This unique material is rich in cellular components both epithelial and stromal cells. Pioneering work by Mina Bissell, Ole William Petersen and others has paved the way for culture of human primary cells from tissue derived from reduction mammoplasty [8, 31, 37]. Isolation protocols for different cell types and subsequent improvements of cell culture conditions for the last two decades have accelerated the search for breast epithelial stem cells and increased the knowledge on how the microenvironment regulates cell fate decision [7, 17, 38]. Initial separation of luminal epithelial- and myoepithelial cells and subsequent findings that subpopulation of luminal epithelial cells could give rise to myoepithelial cells was an important step [37]. In a following paper, it was demonstrated that MUC1 negative and EpCAM positive suprabasal cells had stem cell properties. Isolated MUC1 negative and EpCAM positive suprabasal cells were immortalized using retroviral constructs containing the E6 and E7 oncogenes from the human papilloma virus 16. This cell line was initially referred to as suprabasal cell line [5, 6] and later renamed as D492 [39]. D492 has stem cell properties as measured by its ability to form luminal- and myoepithelial cells in monolayer culture and to generate branching colonies akin to TDLUs when cultured in three-dimensional reconstituted basement membrane (3D-rBM) (Fig. 1). Interestingly, co-expression of both luminal and myoepithelial keratins (K14 and K19) is found in subpopulations of D492 cells. Co-expression of these two keratins is also found in suprabasal cells of the breast gland in situ [6, 40] and has been associated with stem cell properties. Although, D492 cells show stem cell properties by giving rise to both luminal- and myoepithelial cells they are not able to give rise to estrogen receptor (ER) positive luminal epithelial cells. It has been a major problem for decades to culture ER positive normal breast epithelial cells. The only ER positive cell lines available are derived from breast cancer such as MCF-7 and T47-D [41, 42]. Overcoming this problem is important due to the fact that 70% of breast cancers are ER positive and it is currently unknown if this type of breast cancer originates in ER positive cells or if its expression is turned on during cancer progression. Most recently, an important milestone was reached, when Fridriksdottir et al. succeeded in expanding ER positive primary cells in culture. This was achieved by inhibiting the TGFβ receptor with two small molecule inhibitors and by adaption to specific cell culture media [38]. In a following paper, the same group succeeded in generating immortalized normal-derived ER positive cell line referred to as iHBECERpos [43]. Thus, the iHBECERpos cell line and D492 are important normal-derived breast epithelial cell lines that can be highly useful to study normal breast morphogenesis and to contribute to better understanding of ER positive and ER negative breast cancer, respectively.
Application of D492 to Explore EMT and Branching Morphogenesis
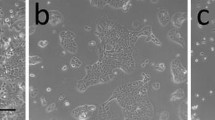
One of the greatest advantages of 3D culture is the recapitulation of morphogenesis in vitro through the ability of cells to interact with each other. One important platform is to create co-culture conditions between epithelial and stromal cells. It has been shown in a number of studies that stroma confers instructive signals to epithelial cells [1, 44, 45]. Stroma is a broad term encompassing extracellular matrix and all the resident cell types that inhabit the stroma, including fibroblasts, immune cells and endothelial cells. Fibroblasts have been shown to be important instructors of morphogenesis in organogenesis, including the mammary gland [46, 47]. Morsing et al. demonstrated recently that fibroblasts in stroma surrounding TDLU in the breast differ greatly from fibroblasts around ducts. Based on marker expression using CD26 (ductal fibroblasts) and CD105 (TDLU associated fibroblasts) as surrogate markers the researchers were able to isolate and characterize these two distinct cell populations. TDLU associated fibroblasts expressed a fibrotic profile in contrast to more immunogenic profile in fibroblasts around ducts. Furthermore, TDLU associated fibroblasts were much more efficient in stimulating branching morphogenesis compared to their ductal counterpart [17]. Immune cells have also been shown to contribute to breast morphogenesis [48, 49]. Macrophages and eosinophiles do contribute to formation of terminal end buds in mouse mammary glands [49]. In addition, Inteferon gamma (IFNgamma) producing T cells have been shown to negatively regulate mammary gland organogenesis affecting luminal epithelial differentiation [48]. The vasculature in the breast has been thoroughly studied in terms of angiogenesis during cancer progression. However, less focus has been on the contribution of endothelial cells to mammary gland morphogenesis. Using the D492 cell line we have established a co-culture system where D492 cells were cultured with organotypic breast endothelial cells (BRENCs) derived from reduction mammoplasty (Fig. 2a) [39, 50]. It is worth mentioning that isolation and expansion of endothelial cells from the breast gland is a time consuming and difficult task, reflected in the dearth of literature discussing primary breast endothelial cells. When plating endothelial cells on top of rBM they quickly form (within 4–10 h) capillary like networks. This is, an unstable structure that dissolves within 48–72 h [50, 51]. Interestingly, when embedded into 3D-rBM, BRENCs stay as single cells inside the gel and do not proliferate (Fig. 2b). They are, however, metabolically active as evidenced by their ability to take up acetylated low-density lipoprotein [50, 51]. In co-culture, BRENCs stimulate proliferation and branching morphogenesis of D492 cells. This is evident when D492 are seeded at clonal density. In 3D monoculture of D492 seeded at clonal density, no growth occurs. In contrast, in co-culture with BRENCs, large branching structures are seen that are remarkably reminiscent of the terminal duct lobular units (TDLUs) of the breast (Fig. 2c). In addition to enhanced branching, BRENCs induced growth of spindle-like mesenchymal colonies in D492 cells (Fig. 2c). Cells isolated from a mesenchymal structure gave rise to a subline referred to as D492M. D492M has a mesenchymal phenotype, measured by a spindle like phenotype in culture and by marker expression. D492M has lost epithelial markers such as keratins, E-cadherin and transcription factors such as p63. In contrast, D492M has gained mesenchymal marker expression including N-cadherin, Vimentin and alpha smooth muscle actin (Fig. 2d). D492M has acquired a cancer stem cell phenotype measured by increased CD44/CD24 ratio, anchorage independent growth, resistance to apoptosis and increased migration/invasion. However, when D492M cells are transplanted into nude mice, they are non-tumorigenic. Using transwell filters where endothelial cells were cultured on top of filters and D492 in rBM below the filter we demonstrated that EMT was facilitated by soluble factors. Blocking hepatocyte growth factor in the culture media partially inhibited mesenchymal colony formation [39].
Endothelial cells induce branching morphogenesis and EMT in D492. a Microvessels in TDLU. Immunostaining for CD31 shows the vascular rich stroma around the TDLU. Green, staining against CD31. Red, staining against Thy-1. Blue, nuclear staining. Bar = 50 μm. Insert, isolated breast endothelial cells (BRENCs) derived from reduction mammoplasty and cultured in monolayer. Green, staining against CD31. Blue, nuclear staining. Bar = 50 μm. Courtesy Sigurdsson et al. [50]. b BRENCs cultivated on top of rBM (upper) or within (lower). On top of rBM BRENCs form capillary-like structures. In contrast, when cultivated within rBM cells appear as single nonproliferative cells. Bar = 50 μm. Courtesy Inthorsson et al. [51]. c Coculture of BRENCs and D492. BRENCs enhance the branching potential of D492 cells but also induce EMT in a subpopulation of D492 cells. Bar = 50 μm. Courtesy Sigurdsson et al. [39]. d Comparison of the D492 and D492M cell lines. D492 express epithelial markers such as E-cadherin and keratins (K14 and K19). In contrast, D492M lack epithelial markers but expresses N-cadherin an EMT marker. Scale bar 100 μm. Courtesy Sigurdsson et al. [39]
Functional Role of MicroRNAs in EMT and MET
Noncoding RNAs (ncRNAs) have in recent years demonstrated the complexity of biological processes that previously were only considered to be regulated by coding genes. MicroRNAs (miRNAs) a well-known class of ncRNAs are involved in most biological processes both in health and diseases [52, 53]. Among those are epithelial to mesenchymal transition (EMT) and its reverse process mesenchymal to epithelial transition (MET) important processes involved in organ development but are hijacked in some forms of cancer [12]. We have applied D492 and D492M to study the role of miRNAs in EMT and MET. Microarray analysis revealed profound changes in miRNA expression between D492 and D492M [54]. The miR-200 family, miR-203a and miR-205 were among the most downregulated miRNAs in D492M. These miRNAs have been described being highly expressed in epithelial tissue and loss of expression has been shown to occur during EMT [55,56,57]. The miR-200 family is composed of five miRNAs (mir-200a, mir-200b, miR-200c, miR-141 and miR-429). In humans, miR-200c and miR-141 are located on the same cluster on chromosome 12. They share a promoter but their seeding sequence is different. In D492M, overexpression of miR-200c-141 induced MET, but only towards the luminal epithelial phenotype. D492MmiR-200c-141 expresses the epithelial marker E-cadherin and luminal epithelial markers such as EpCAM and K19. However, the expression of myoepithelial markers such as P-cadherin, K14, K5/6, K17 and the transcription factor p63 were not re-induced in the cell line. In 3D culture, D492MmiR-200c-141 retains epithelial phenotype, but does not form branching colonies like D492. In addition, constitutive expression of miR-200c-141 in D492 does not affect its ability to form branching colonies in 3D culture, it does however, prevent endothelial induced EMT. This means that while miR-200c-141 governs epithelial phenotype in the cell line, its downregulation is not necessary for the cells to carry out branching morphogenesis, which involves partial EMT.
The miR-200 family has previously been linked to epithelial integrity and EMT and using the D492 model system we have shown that miR-200c-141 inhibits EMT in epithelial cells and enforces MET in mesenchymal cells to a luminal epithelial phenotype. In order to capture the full phenotype of D492 with both luminal and myoepithelial marker expression and branching morphogenesis we used p63, a well-known transcription factor expressed in myoepithelial cells in the breast. p63 expression did not reoccur when miR-200c-141 was introduced to D492M. Overexpression of p63 together with miR200c-141 in D492M resulted in reinvention of both the luminal- and myoepithelial phenotype and the ability of generating branching structures reminiscent of TDLU in 3D-rBM. These results highlight the importance of cells with both luminal and myoepithelial characteristics for a full branching morphogenesis to take place and underscore the importance of both lineage expression patterns in development (Fig. 3).
Application of D492 and D492M to study EMT and MET. D492 form branching TDLU like structures in 3D-rBM. When cocultured with BRENCs, the branching potential is enhanced but simultaneously a subpopulation of D492 cells undergoes EMT. D492M was established from mesenchymal colonies derived from endothelial induced EMT of D492. When overexpressed in D492M, microRNA-200c-141 was able to revert the EMT phenotype back to the epithelial, albeit only to a luminal epithelial phenotype. Overexpression of P63, an important transcription factor for myoepithelial cells, along with miR-200c-141 were sufficient to revert D492M back to the branching epithelial phenotype seen in D492. Bar = 50 μm
Collectively, the differences in expression of numbers of miRNAs between D492 and D492M makes these isogenic cell lines highly useful to explore miRNA functions in breast epithelial morphogenesis, EMT and MET. So far, we have only been looking into a small selection of miRNAs that are the most downregulated in D492M and we are currently working on other miRNAs in these cell lines.
Oncogene-Induced EMT and Tumor Progression
As mentioned above, D492 has stem cell properties and can undergo EMT through interactions with stromal cells, as evidenced by the ability of endothelial cells to induce EMT in this cell line. Due to the stem cell properties including the ability to form branching structures reminiscent of TDLU when cultured in 3D-rBM we decided to analyze the effects of overexpressing oncogenes in D492 to see if this would push the cells towards increased tumorigenicity. We chose EGFR and EGFR2 (HER2) due to their common overexpression and/or amplification in breast cancers [58] (Fig. 4a). D492 being a predominantly basal cell line, expresses high levels of endogenous EGFR. In contrast HER2 is not expressed in D492, consistent with the fact that HER2 is predominantly expressed in luminal epithelial cells in the breast [59]. When, EGFR was overexpressed in D492 we saw an increase in the basal/myoepithelial phenotype. Interestingly HER2 overexpressing D492 cells underwent EMT and formed disorganized spindle-shape or grape like colonies in 3D culture (Fig. 4b). In addition, overexpression of HER2 and the resulting EMT caused a downregulation of endogenous expression of EGFR. This could be partially reverted by ectopic expression of EGFR in the HER2 expressing cells (double expression). In monolayer, cells expressing HER2 exhibited an EMT phenotype, with or without expression of EGFR. However, in 3D culture, E-cadherin expression could be seen in cultures of HER2/EGFR cells, indicating that EGFR expression was sufficient to revert the HER2 induced EMT under the correct culture conditions.
HER2 overexpression in D492 induces tumorigenesis. a Overexpression of HER2 in D492 reduces the endogenous expression of EGFR. b D492HER2 generate grape-like or disorganized spindle-like colonies in 3D-rBM. c D492HER2 generate large tumors when transplanted into nude mice. Cotransfection of HER2 and EGFR in D492 (D492HER2/EGFR) reduces the tumor growth. D492EGFR does not form tumors in mice. D) Treating D492HER2 cells with combination of traztuzmab (HER2 antibody) and Cetuximab (EGFR antibody) results in poorer outcome than treated with traztuzumab alone. Bar = 50 μm. Courtesy Ingthorsson et al. [59]
When transplanted into nude mice D492HER2 cells formed large tumors. Interestingly D492EGFR/HER2 were not as tumorigenic as D492HER2. While all mice developed tumors, the growth was stunted compared to HER2 alone, indicating that EGFR has some growth suppressive properties in D492EGFR/HER2 (Fig. 4c). Incidentally, we observed that the HER2/EGFR tumors had high levels of epithelial cells, consistent with the 3D culture observations. We therefore postulated that EGFR enabled the HER2 overexpressing cells to maintain an epithelial phenotype, resulting in slower tumor growth. Finally, we treated D492HER2 tumor xenografted mice with either trastuzumab (monoclonal antibody against HER2), cetuximab (monoclonal antibody against EGFR) or combination of both for 20 days. As expected, treatment with trastuzumab reduced the volume of the D492HER2 tumors. Cetuximab resulted in growth arrest during treatment. Combination of trastuzumab and cetuximab also reduced the tumor volume initially but after end of treatment tumor growth resumed. Most interestingly, if the mice with the D492HER2 tumors were treated with cetuximab and trastuzumab, advanced growth was seen compared to treatment with trastuzumab alone, indicating that EGFR treatment in a HER2 driven environment could have detrimental effects (Fig. 4d) [59]. Although, considered negative for EGFR based on immunostaining, D492HER2 cells were positive for phosphorylated EGFR when tested on western blot. Thus, these data indicate that EGFR expression may work as a tumor suppressor in the D492HER2 cells and combination treatment with both trastuzumab and cetuximab could actually override the effects achieved by treatment with trastuzumab alone.
D492HER2 cells show mesenchymal traits, but not as prominent as D492M. It is possible that partial EMT in D492HER2 renders the cells with increased cellular plasticity that further accelerates the tumor growth. In contrast D492M is more fixed in its mesenchymal state, possibly explaining its low tumorigenicity. In a recent review, Shibue et al. discussed cellular plasticity and EMT [12]. Herein they argue that tumor cells were highly malignant if they were showing partial EMT but not if their phenotype was more into the mesenchymal differentiation [12]. It is possible that partial EMT is reflecting cellular plasticity rather than true EMT and that these cells can change their phenotype in order to adapt better to new environments, which is necessary for cancer cell invasion and metastasis.
Concluding Remarks and Future Perspectives
Although animals (mainly mice) have contributed significantly to research on mammary gland morphogenesis and cancer it is of importance to acknowledge that there is a histological and biological difference between the mouse mammary gland and the human female breast that cannot be ignored. Data is accumulating that in the human breast the TDLU-associated stroma has a dominant role on branching morphogenesis. TDLU in addition to its milk producing function during lactation is the place where most breast cancers originate. In mice, there are no TDLUs, only terminal end buds and the stroma is mainly composed of fat tissue. If the stroma is dominant in bringing signals to the epithelium it is obvious that these signals are different between mice and humans. It is also important to acknowledge that cell culture is and will continue to be conducted in an artificial environment. However, using breast cells, both epithelial and stromal cells, and continuous improvement of cell culture environment will help to fill the gaps that exist between the mouse and the human mammary gland. There is no doubt that cell lines are important and will continue to be so in the near future. They are important tools but have their disadvantages that need to be taken into account when experiments are designed.
References
Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125.
Javed A, Lteif A. Development of the human breast. Semin Plast Surg. 2013;27:5–12.
Arendt LM, Kuperwasser C. Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy. J Mammary Gland Biol Neoplasia. 2015;20:9–25.
Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142:1028–42.
Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706.
Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101.
Fridriksdottir AJ, Villadsen R, Morsing M, Klitgaard MC, Kim J, Petersen OW, et al. Proof of region-specific multipotent progenitors in human breast epithelia. Proc Natl Acad Sci U S A. 2017;114(47):E10102–E10111. https://doi.org/10.1073/pnas.1714063114.
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50.
Miyano M, Sayaman RW, Stoiber MH, Lin CH, Stampfer MR, Brown JB, et al. Age-related gene expression in luminal epithelial cells is driven by a microenvironment made from myoepithelial cells. Aging (Albany NY). 2017;9:2026–51.
Nelson AC, Machado HL, Schwertfeger KL. Breaking through to the other side: microenvironment contributions to DCIS initiation and progression. J Mammary Gland Biol Neoplasia. 2018;23:207–21.
Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–72.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Borowsky AD. Choosing a mouse model: experimental biology in context--the utility and limitations of mouse models of breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a009670.
Asselin-Labat ML, Vaillant F, Shackleton M, Bouras T, Lindeman GJ, Visvader JE. Delineating the epithelial hierarchy in the mouse mammary gland. Cold Spring Harb Symp Quant Biol. 2008;73:469–78.
Cardiff RD, Bern HA, Faulkin LJ, Daniel CW, Smith GH, Young LJ, et al. Contributions of mouse biology to breast cancer research. Comp Med. 2002;52:12–31.
Morsing M, Klitgaard MC, Jafari A, Villadsen R, Kassem M, Petersen OW, et al. Evidence of two distinct functionally specialized fibroblast lineages in breast stroma. Breast Cancer Res. 2016;18:108.
Dontu G, Ince TA. Of mice and women: a comparative tissue biology perspective of breast stem cells and differentiation. J Mammary Gland Biol Neoplasia. 2015;20:51–62.
Cardiff RD, Wellings SR. The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia. 1999;4:105–22.
Koledova Z. 3D cell culture: an introduction. Methods Mol Biol. 1612;2017:1–11.
Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017;216:31–40.
Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–28.
Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985;82:1419–23.
Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–55.
Linnemann JR, Meixner LK, Miura H, Scheel CH. An organotypic 3D assay for primary human mammary epithelial cells that recapitulates branching morphogenesis. Methods Mol Biol. 1612;2017:125–37.
Linnemann JR, Miura H, Meixner LK, Irmler M, Kloos UJ, Hirschi B, et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development. 2015;142:3239–51.
Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20.
Lotan D. Reconstituted basement membrane (matrigel®): a useful semisolid medium for growth of tumor cell colonies. In Vitro Cell Dev Biol. 1996;32:192–3.
Anonymous. Matrigel®basement membrane matrix. Beckton Dickinson Technical letter 1995;7/95-CBP-GFRMATRIGEL.
Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35.
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–8.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–86 e10.
Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, Gupta PB. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18:19.
dos Santos CO, Rebbeck C, Rozhkova E, Valentine A, Samuels A, Kadiri LR, et al. Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc Natl Acad Sci U S A. 2013;110:7123–30.
Fu NY, Rios AC, Pal B, Law CW, Jamieson P, Liu R, et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat Cell Biol. 2017;19:164–76.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28:1143–58.
Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999;206:88–99.
Fridriksdottir AJ, Kim J, Villadsen R, Klitgaard MC, Hopkinson BM, Petersen OW, et al. Propagation of oestrogen receptor-positive and oestrogen-responsive normal human breast cells in culture. Nat Commun. 2015;6:8786.
Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, Fridriksdottir AJ, Ringner M, Villadsen R, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS One. 2011;6:e23833.
Villadsen R. In search of a stem cell hierarchy in the human breast and its relevance to breast cancer evolution. APMIS. 2005;113:903–21.
Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973;248:6251–3.
Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215.
Hopkinson BM, Klitgaard MC, Petersen OW, Villadsen R, Ronnov-Jessen L, Kim J. Establishment of a normal-derived estrogen receptor-positive cell line comparable to the prevailing human breast cancer subtype. Oncotarget. 2017;8:10580–93.
Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–46.
Hansen RK, Bissell MJ. Tissue architecture and breast cancer: the role of extracellular matrix and steroid hormones. Endocr Relat Cancer. 2000;7:95–113.
Sadlonova A, Novak Z, Johnson MR, Bowe DB, Gault SR, Page GP, et al. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 2005;7:R46–59.
Medina D. Stromal fibroblasts influence human mammary epithelial cell morphogenesis. Proc Natl Acad Sci U S A. 2004;101:4723–4.
Plaks V, Boldajipour B, Linnemann JR, Nguyen NH, Kersten K, Wolf Y, et al. Adaptive immune regulation of mammary postnatal organogenesis. Dev Cell. 2015;34:493–504.
Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82.
Sigurdsson V, Fridriksdottir AJ, Kjartansson J, Jonasson JG, Steinarsdottir M, Petersen OW, et al. Human breast microvascular endothelial cells retain phenotypic traits in long-term finite life span culture. In Vitro Cell Dev Biol Anim. 2006;42:332–40.
Ingthorsson S, Sigurdsson V, Fridriksdottir AJ, Jonasson JG, Kjartansson J, Magnusson MK, et al. Endothelial cells stimulate growth of normal and cancerous breast epithelial cells in 3D culture. BMC Res Notes. 2010;3:184.
Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–24.
Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7.
Hilmarsdottir B, Briem E, Sigurdsson V, Franzdottir SR, Ringner M, Arason AJ, et al. MicroRNA-200c-141 and Np63 are required for breast epithelial differentiation and branching morphogenesis. Dev Biol. 2015;403:150–61.
Moes M, Le Bechec A, Crespo I, Laurini C, Halavatyi A, Vetter G, et al. A novel network integrating a miRNA-203/SNAI1 feedback loop which regulates epithelial to mesenchymal transition. PLoS One. 2012;7(4):e35440. https://doi.org/10.1371/journal.pone.0035440.
Tran MN, Choi W, Wszolek MF, Navai N, Lee IL, Nitti G, et al. The p63 protein isoform DeltaNp63alpha inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem. 2013;288:3275–88.
Hilmarsdottir B, Briem E, Bergthorsson JT, Magnusson MK, Gudjonsson T. Functional role of the microRNA-200 family in breast morphogenesis and neoplasia. Genes. 2014;5:804–20.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Ingthorsson S, Andersen K, Hilmarsdottir B, Maelandsmo GM, Magnusson MK, Gudjonsson T. HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells is inhibited by coexpression of EGFR. Oncogene. 2016;35(32):4244–55. https://doi.org/10.1038/onc.2015.489.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Briem, E., Ingthorsson, S., Traustadottir, G.A. et al. Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture. J Mammary Gland Biol Neoplasia 24, 139–147 (2019). https://doi.org/10.1007/s10911-018-09424-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-018-09424-w