Abstract
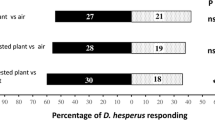
The ability of female parasitoid wasps, Cotesia kariyai (Hymenoptera: Braconidae), to learn to recognize plant volatiles associated with an oviposition experience was investigated. First, we observed oviposition behavior of female wasps when encountering host larvae (Mythimna separata, Lepidoptera: Noctuidae). On their first encounters with host larvae, 96% of inexperienced female wasps showed oviposition behavior. Immediately afterward, and for 2 h after the first oviposition, wasp oviposition behavior was suppressed. Only 20–30% of wasps showed a second oviposition response, even after several encounters with a new host. Then, oviposition behavior gradually recovered 6 to 24 h after the first oviposition. At 6 h after the first oviposition experience, more than 80% of the wasps showed a second oviposition response when encountering a new host. Next, in a wind tunnel, we investigated the effect of time since first oviposition on wasp responses to host-infested plant volatiles. Female landings in response to volatiles of host-infested maize plants, Zea mays, showed a bell-shaped curve from 0 to 48 h after first oviposition. Eight hours after the first oviposition, the rate of landing by wasps was significantly higher than that of oviposition-inexperienced wasps. The ecological strategy explaining the effect of oviposition experience on olfactory responses of C. kariyai to host-infested plant volatiles is discussed.


Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Afsheen S, Wang X, Li R, Zhu C, Lou Y (2008) Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci 15:381–397
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nat Rev Neurosci 3:542–552
Dukas R (2008) Evolutionary biology of insect learning. Annu Rev Entomol 53:145–160
Dukas R (2013) Effects of learning on evolution: robustness, innovation and speciation. Anim Behav 85:1023–1030
Dutton A, Mattiacci L, Dorn S (2000) Learning used as a strategy for host stage location in an endophytic host-parasitoid system. Entomol Exp Appl 94:123–132
Fukushima J, Kainoh Y, Honda H, Takabayashi J (2001) Learning of host-infested plant volatiles in the larval parasitoid Cotesia kariyai. Entomol Exp Appl 99:341–346
Fukushima J, Kainoh Y, Honda H, Takabayashi J (2002) Learning of herbivore-induced and nonspecific plant volatiles by a parasitoid, Cotesia kariyai. J Chem Ecol 28:579–586
Girling RD, Hassall M, Turner JG, Poppy GM (2006) Behavioural responses of the aphid parasitoid Diaeretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol Exp Appl 120:1–9
Giunti G, Canale A, Messing RH, Donati E, Stefanini C, Michaud JP, Benelli G (2015) Parasitoid learning: current knowledge and implications for biological control. Biol Control 90:208–219
Godfray HCJ (1994) Parasitoids behavioral and evolutionary ecology. Princeton University Press, Princeton
Hirai K (1984) Notes on Apanteles kariyai Watanabe (Hymenoptera: Braconidae), a parasitoid of the armyworm Pseudaletia separata Walker. Ann Rep Plant Prot North Jpn 35:154–156
Kaiser L, Pérez-Maluf R, Sandoz JC, Pham-Delègue MH (2003) Dynamics of odour learning in Leptopilina boulardi, a hymenopterous parasitoid. Anim Behav 66:1077–1084
Kanda K (1988) Hatching time of eggs and dispersal time of resultant hatching of the army-worm, Pseudaletia separata Walker. Jap J Appl Entomol Zool 32:85–87 (in Japanese with an English summary)
Kanda K (1991) Rearing method of armyworm. In: Yushima K, Kamano S, Tamaki Y (eds) Rearing method of insects. Japan Plant Protection Association, Tokyo, pp 206–209 (in Japanese)
Kanda K, Naito A (1979) Behavior of oriental armyworm moth, Leucania separata Walker, from emergence to oviposition. Jap J Appl Entomol Zool 23:69–77 (in Japanese with an English summary)
Lewis WJ, Martin WR (1990) Semiochemicals for use with parasitoids: status and future. J Chem Ecol 16:3067–3089
Mangel M (1993) Motivation, learning and motivated learning. In: Papaj DR, Lewis AC (eds) Insect learning - ecological and evolutionary perspectives. Chapman& Hall, London, pp 158–173
Nealis VG (1986) Responses to host kairomones and foraging behavior of the insect parasite Cotesia rubecula (Hymenoptera: Braconidae). Can J Zool 64:2393–2398
Quicke DLJ (2015) Host location, associative learning and host assessment. In: Quicke DLJ (ed) The braconid and ichneumonid parasitoid wasps: biology, systematics, evolution and ecology. John Wiley & Sons, Chichester
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-proje
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Sato Y, Tanaka T (1984) Effect of the number of parasitoid (Apanteles kariyai) eggs (Hym.: Braconidae) on the growth of host (Leucania separata) larvae (Lep.: Noctuidae). Entomophaga 29:21–28
Smid HM, Wang G, Bukovinszky T, Steidle JLM, Bleeker MAK, van Loon JJA, Vet LEM (2007) Species-specific acquisition and consolidation of long-term memory in parasitic wasps. P Roy Soc B: Biol Sci 274:1539–1546
Takabayashi J, Shiojiri K (2019) Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Curr Opin Insect Sci 32:110–117
Takasu K, Lewis WJ (1996) The role of learning in adult food location by the larval parasitoid, Microplitis croceipes (Hymenoptera: Braconidae). J Insect Behav 9:265–281
Takemoto H, Powell W, Pickett J, Kainoh Y, Takabayashi J (2009) Learning is involved in the response of parasitic wasps Aphidius ervi (Haliday) (Hymenoptera: Braconidae) to volatiles from a broad bean plant, Vicia faba (Fabaceae), infested by aphids Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Appl Entomol Zool 44:23–28
Takemoto H, Powell W, Pickett J, Kainoh Y, Takabayashi J (2012) Two-step learning involved in acquiring olfactory preferences for plant volatiles by parasitic wasps. Anim Behav 83:1491–1496
Tanaka A (1976) Population dynamics in Mythimna separata (Walker). Plant Prot 30:431–437 (in Japanese)
Tanaka T, Agui N, Hiruma K (1987) The parasitoid Apanteles kariyai inhibits pupation of its host, Pseudaletia separata, via disruption of prothoracicotropic hormone release. Gen Comp Endocrinol 67:364–374
Tanaka T, Matsumoto H, Hayakawa Y (2002) Analysis in the course of polydnavirus replication in ovarian calyx cells of the parasitoid wasp, Cotesia kariyai (Hymenoptera: Braconidae). Appl Entomol Zool 37:323–328
Tempel BL, Bonini N, Dawson DR, Quinn WG (1983) Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A 80:1482–1486
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452
Turlings TCJ, Wäckers FL, Vet LEM, Lewis WJ, Tumlinson JH (1993) Learning of host-finding cues by hymenopterous parasitoids. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspective. Chapman and Hall, New York, pp 51–78
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in tritrophic context. Annu Rev Entomol 37:141–172
Vet LEM, Lewis WJ, Cardé RT (1995) Parasitoid foraging and learning. In: Cardé RT, Bell WJ (eds) Chemical ecology of insects 2. Chapman and Hall, New York, pp 65–101
Villar ME, Marchal P, Viola H, Giurfa M (2020) Redefining single-trial memories in the honeybee. Cell Rep 30:2603–2613
Vos M, Vet LEM (2004) Geographic variation in host acceptance by an insect parasitoid: genotype versus experience. Evol Ecol Res 6:1021–1035
Wäschke N, Meiners T, Rostas M (2013) Foraging strategies of parasitoids in complex chemical environments. In: Wajnberg E, Colazza S (eds) Chemical ecology of insect parasitoids. Wiley-Blackwell, Chichester, pp 37–63
Acknowledgements
We thank Prof. DeMar Taylor, Faculty of Life and Environmental Sciences, University of Tsukuba, for his comments on the early version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
There are no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fukushima, J., Kuramitsu, K., Takabayashi, J. et al. Delayed Response after Learning Associated with Oviposition Experience in the Larval Parasitoid, Cotesia kariyai (Hymenoptera: Braconidae). J Insect Behav 34, 264–270 (2021). https://doi.org/10.1007/s10905-021-09786-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-021-09786-w