Abstract
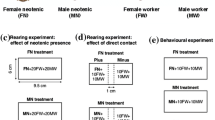
In lower termites, functionally sterile larval helpers are totipotent—capable of becoming reproductively active with the loss of their colony’s king or queen. Full reproductive development may take several weeks, but initiation of this developmental response most likely occurs shortly after colony members detect when a reproductive-specific signal is missing. We investigated the early response of termite helpers to the removal of their king and queen in the basal termite species Zootermopsis nevadensis. Within 6–12 h after reproductives were removed, helpers displayed an increase in head-butting—a behavior associated with dominance in other termite species as well as in closely related roaches. The loss of just one reproductive, either the king or queen, was also sufficient to cause an increase in head-butting. We did not find evidence, however, that this response was sex-specific: males and females were equally likely to increase head-butting independent of the sex of the reproductive that was removed. Finally, we discovered that reproductive-specific compounds present on the cuticle of king and queen termites were also present in their feces, but the presence of the feces did not seem sufficient to inhibit the increased head-butting after the reproductives were removed. Collectively, these results indicate that termite workers readily detect the loss of reproductives in their colony and that they at least initially respond in a non sex-specific manner.



Similar content being viewed by others
References
Bell W, Gorton R, Tourtellot M, Breed M (1979) Comparison of male agonistic behavior in five species of cockroaches. Insectes Soc 26:252–263
Bordereau C, Lacey MJ, Sémon E, Braekman JC, Ghostin J, Robert A, Shellman Sherman J, Sillam-Dussès D (2010) Sex pheromones and trail-following pheromone in the basal termites Zootermopsis nevadensis (Hagen) and Z. angusticollis (Hagen) (Isoptera: Termopsidae: Termopsinae). Biol J Linn Soc 100:519–530
Brent CS, Traniello JFA (2001) Influence of sex specific stimuli on ovarian maturation in primary and secondary reproductives of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 26:239–247
Brent CS, Schal C, Vargo EL (2007) Endocrine effects of social stimuli on maturing queens of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 32:26–33
Castle G (1934) The damp-wood termites of western United States, genus Zootermopsis (formerly, Termopsis). In: Kofoid C (ed) Termites and termite control. University of California Press, Berkeley, pp 264–282
Clark DC, Moore AJ (1994) Social interactions and aggression among male Madagascar hissing cockroaches (Gromphadorhina portentosa) in groups (Dictyoptera: Blaberidae). J Insect Behav 7:199–215
Costa-Leonardo AM, Arab A, Casarin FE (2004) Neotenic formation in laboratory colonies of the termite Coptotermes gestroi after orphaning. J Insect Sci 4:10
Ewing L (1967) Fighting and death from stress in a cockroach. Science 155:1035–1036
Greenberg SLW, Stuart A (1982) Precocious reproductive development (neoteny) by larvae of a primitive termite Zootermopsis angusticollis (Hagen). Insectes Soc 29:535–547
Hanus R, Vrkoslav V, Hrdy I, Cvacka J, Sobotník J (2010) Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc Roy Soc B 277:995–1002
Haverty MI, Woodrow RJ, Nelson LJ, Grace JK (2005) Identification of termite species by the hydrocarbons in their feces. J Chem Ecol 31:2119–2151
Heath H (1903) The habits of California termites. Biol Bull 4:47–63
Inward D, Beccaloni G, Eggleton P (2007) Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Let 3:331–335
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794
Kirchner WH, Broecker I, Tautz J (1994) Vibrational alarm communication in the damp-wood termite Zootermopsis nevadensis. Physiol Entomol 19:187–190
Korb J, Weil T, Hoffmann K, Foster KR, Rehli M (2009) A gene necessary for reproductive suppression in termites. Science 324:758
Le Conte Y, Hefetz A (2008) Primer pheromones in social hymenoptera. Ann Rev Entomol 53:523–542
Liebig J (2010) Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In: Blomquist GJ, Bagnères AG (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, New York, pp 254–281
Liebig J, Eliyahu D, Brent C (2009) Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol 63:1799–1807
Light SF (1943) The determination of the castes of social insects (concluded). Quart Rev Biol 18:46–63
Light SF, Weesner FM (1951) Further studies on the production of supplementary reproductives in Zootermopsis (Isoptera). J Exper Zool 117:397–414
Lüscher M (1955) Zur Frage der Übertragung sozialer Wirkstoffe bei Termiten. Naturwissenschaften 42:186–186
Matsuura K, Yamamoto Y (2011) Workers do not mediate the inhibitory power of queens in a termite, Reticulitermes speratus (Isoptera, Rhinotermitidae). Insect Soc 58:513–518
Matsuura K, Himuro C, Yokoi T, Yamamoto Y, Vargo E, Keller L (2010) Identification of a pheromone regulating caste differentiation in termites. Proc Nat Acad Sci USA 107:12963–12968
Mensa-Bonsu A (1976) The production and elimination of supplementary reproductives in Porotermes adamsoni (Froggatt). Insectes Soc 23:133–154
Monnin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fennici 43:515–530
Myles TG (1999) Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiol 33:1–94
Nagin R (1972) Caste determination in Neotermes jouteli (Banks). Insectes Soc 19:39–61
Pasteels JM (1972) Sex-specific pheromones in a termite. Cell Molec Life Sci 28:105–106
Peeters C, Liebig J (2009) Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. In: Gadau J, Fewell J (eds.) Organization of Insect Societies: From Genome to Socio-Complexity. Harvard University Press, pp 220–242
Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA (1999) Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86:544–548
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton
Stuart A (1963) Studies on the communication of alarm in the termite Zootermopsis nevadensis (Hagen), Isoptera. Physiol Zool 36:85–96
Stuart A (1979) The determination and regulation of the neotenic reproductive caste in the lower termites (Isoptera): with special reference to the genus Zootermopsis (Hagen). Sociobiol 4:223–237
Thorne BL (1996) Termite terminology. Sociobiol 28:253–263
Weil T, Hoffmann K, Kroiss J, Strohm E, Korb J (2009) Scent of a queen: cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften 96:315–319
Acknowledgements
We would like to thank Kevin Haight for assistance with colony collection and the administrators of the Pebble Beach Company for permission to collect termites. Additionally, we would like to thank Steve Prager and Barbara Birtcil for assistance with colony maintenance and experiments. All experiments were conducted in accordance with American statutes governing research. This project was supported by the Agriculture and Food Research Initiative Competitive Grant no. 2007-35302-18172 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
Footage providing examples of head-butting behavior and head-banging (alarm) behavior. Head-butting consists of a single forward motion directed towards another individual while head-banging is a repeated up and down motion of the head that is not usually directed at another individual. (M1V 2545 kb)
Rights and permissions
About this article
Cite this article
Penick, C.A., Trobaugh, B., Brent, C.S. et al. Head-butting as an Early Indicator of Reproductive Disinhibition in the Termite Zootermopsis nevadensis . J Insect Behav 26, 23–34 (2013). https://doi.org/10.1007/s10905-012-9332-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-012-9332-x