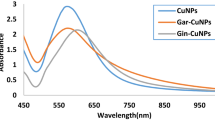
Abstract
Herein, synthesis and characterization of hybrid nanoflowers (hNFs) using gallic acid as organic components, and Cu(II), and Zn(II) ions which were involved as the inorganic component was carried out. In the first part of the study, the morphologies of the synthesized hNFs were investigated using SEM, EDX, XRD, FT-IR, and mapping. In the second part of the study, the antimicrobial activities of the synthesized hNFs were examined. Antimicrobial activities of hNFs were systematically studied against Staphylococcus aureus, Escherichia coli, P. aeruginosa, and Candida albicans with broth microdilution and short time-kill assay. In addition, the anticancer activity of hNFs was examined. For this, A549 and MCF7 cell lines were used.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a promising and rapidly developing field of science that manipulates matter in the nanoscale range, which has so far made outstanding contributions to technological innovations, including the development of advanced properties and adaptable nanoscale materials (nanomaterials) in the biomedical field [1,2,3]. Among the different nanomaterials developed so far, nanoparticles have attracted great attention due to their unique properties, especially the ability to modify catalytic, thermal, and mechanical properties, and increased surface-to-volume ratio [4]. Furthermore, the interest in nanoparticles may be a result of their properties such as size, morphology, surface charge, and stability, as well as their wide range of applications [5,6,7]. These properties make nanoparticles useful in pharmaceutical and medical fields, such as improved drug delivery, drug-controlled release, and diagnostic applications. It has even made it very interesting in the field of cosmetics [8] Metal-based nanoparticles (MNPs) consist of metals (metal nanoparticles) or metallic compounds (metal oxide nanoparticles).
In general, due to their remarkable properties, including small size, metallic composition, and high surface area, MNPs have been widely used in many science applications, such as biological and medical fields, beyond their use in optics, electronics, magnetic fields [9,10,11].
In recent years, green chemistry methods have been developed for the synthesis of metal nanoparticles [12,13,14,15]. The main aim of these studies is to find an environmentally friendly and practical technique for good characterization and production of nanomaterials. Nanoparticles have unique properties [16,17,18]. They are used in a wide variety of applications such as bioimaging, drug delivery, anticancer, antimicrobial, and catalytic agents. Nanoflowers (NFs), consisting of a combination of organic and inorganic materials, are a new class of nanoparticles developed in recent years [19,20,21]. The structures of Nfs attract a lot of attention today due to their increased surface area, stability, and efficiency [22,23,24].
Various nanostructures can be synthesized using various materials such as protein, DNA, polymer, metal, and plant. For the first time, Ge et al. made an important discovery in the formation of flower-shaped hybrid nanostructures containing enzymes (organic matter) and copper phosphate (inorganic matter) and introduced them to the scientific world [25]. Although initially called “Nfs” by this group because they consist of nanometer-sized petals, the hybrid flower-shaped structures are micrometer-sized. In addition, in many studies conducted in recent years, it has been shown that organic molecules containing OH groups, as well as amine and carboxyl groups and plant extracts, are used in nanoflower synthesis [26].
In addition, nanoflower structures have many advantages such as good stability, large surface area, low toxicity, and environmental friendliness [27].
Generally, most organic–inorganic hybrid nanoflowers (hNFs) have been synthesized with copper ions. The synthesized copper phosphate hNFs exhibit higher catalytic activity and stability. The increased activity in copper phosphate hNFs could possibly be due to the following factors [18]: (1) high surface area of Nfs; this reduces mass transfer limitations by (2) the active effects of copper in nanoscale immobilized organic molecules and (3) the mutual influence of the organic molecule and metal ions on each other.
Gallic acid (3,4,5-trihydroxybenzoic acid), a well-known polyphenol, is an important consideration in this respect as it is selectively cytotoxic against various tumor cells [17]. Gallic acid is an endogenous plant polyphenol found abundantly in wine, as well as in tea, grapes, nuts, and other fruits. [6]. Gallic acid, known to affect various pharmacological and biochemical pathways, has strong antioxidant, anti-inflammatory, antimutagenic, and anticancer properties. It is also known that gallic acid has pro-oxidant properties depending on concentration in the presence of metal ions [28]. The pro-oxidant property of gallic acid has been recognized as an inducer of apoptosis in cancer cell lines. Studies have also confirmed the protective effect of gallic acid in chemically induced carcinogenesis. Gallic acid has different effects on various tumor types at different molecular levels [29].
Its anti-cancer effects make this compound an important biomolecule for therapeutic uses. Gallic acid has shown selective cytotoxicity for cancerous cells and has much less toxicity for normal cells [30]. This property makes gallic acid a valuable additive along with vitamins and is used as a nutritional supplement to prevent cancer risks [31].
Huang et al. (2023) examined the elimination of Gram-positive bacteria by performing gallic acid–Zn hNF synthesis [32]. Dadi et al. (2020) carried out a peroxidase-like activity study for the determination of m-cresol by performing gallic acid–Cu hNF synthesis [33].
Although there are many studies on hNF synthesis, their anticancer activities are just being investigated. There are very few studies on this. To give an example, Somtürk et al. (2024) synthesized hNFs using Tribulus terrestris L. extract as the organic part and Cu, Co and Zn metal ions as the inorganic part. They used SEM, EDX, XRD, FTIR for the characterization of the synthesized hNFs. Additionally, they tested the anticancer activities of hNFs on the A549 cell line. They observed that the anticancer activity was greatly increased when the plant extract was converted into hNF form [34].
Based on this study, the anticancer activities of previously synthesized gallic acid hNFs were tested on A549 and MCF7 cell lines. Additionally, the antimicrobial activities of the hNFs to be synthesized were investigated.
2 Materials and Methods
2.1 Chemicals and Reagents
Copper (II) sulfate pentahydrate (CuSO4·5H2O) was purchased from Sigma-Aldrich (USA). Potassium phosphate dibasic (KH2PO4), calcium chloride dihydrate (CaCl2·2H2O), magnesium chloride (MgCl2·6H2O), sodium chloride (NaCl), potassium chloride (KCl), sodium phosphate (Na2HPO4) were obtained from Sigma-Aldrich and utilized for the synthesis of the pH solutions. Ultrapure water was used to prepare aqueous solutions throughout the work.
2.2 Synthesis of Gallic Acid–Copper (II) Hybrid Nanoflowers (Gallic Acid–CuhNFs)
Gallic acid–CuhNFs synthesis was previously described by Somturk et al. It was synthesized using a method reported by [34,35,36,37]. A certain volume of the prepared CuSO4·5H2O (120 mM) stock solution was added to 8 mL of 10 mM PBS (pH:7.4) solution. Then, gallic acid was added to the medium at a concentration of 0.02 mg/mL. These mixtures were vortexed for 20 s and then incubated at 4 °C for 3 days. Afterwards, the obtained mixture was centrifuged at 6500 rpm for 15 min and the supernatant part was discarded and the remaining pellet part was left to dry at room temperature. The gallic acid–CuhNFs synthesis scheme is seen in Fig. 1.
2.3 Synthesis of Gallic Acid–Zinc (II) Hybrid Nanoflowers (Gallic Acid–ZnhNFs)
Hybrid nanoflowers containing Zn (II) ions were synthesized based on the method reported by Zhang et al. (2016), with some modifications [34]. In this method; first, Zn(CH3COO)2·2H2O (18.3 mM) was prepared. Then, 16 mg gallic acid and a certain volume of Zn (II) ions was added to 20 mL phosphate buffer (pH 7.4) in separate tubes. The mixture was kept in the magnetic stirrer for 24 h at 25 °C. After incubation, it was centrifuged at 6500 rpm for 15 min. Finally, the collected ZnhNFs were dried at 25 °C. The schematic representation of the synthesis of Nfs containing Zn (II) ions is given in Fig. 2.
2.4 Characterization of Gallic Acid–CuhNFs and Gallic Acid–ZnhNFs
The morphologies of synthesized gallic acid–CuhNFs and gallic acid–ZnhNFs were determined using scanning electron microscopy (FESEM, Zeiss GeminiSEM 500), while the weight and atomic percentages of Cu, Zn, P, O, and Cl elements in the CuhNFs structure were determined using energy dispersive X-ray (EDX). The crystal structures of hNFs were elucidated using X-ray diffraction analysis (XRD) BRUKER AXS D8). The chemical structure of the Nfs was carried out using the FT-IR (Perkin Elmer Spectrum 400) spectrum. Size analysis of hNFs was determined using the Image ProPlas 6.0 program.
2.5 Antimicrobial Activity Assay
Minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) assays were studied according to clinical laboratory standards. Gram-negative (Escherichia coli ATCC 25923, Pseudomonas aeruginosa ATCC 27853), Gram-positive (Staphylococcus aureus ATCC 25923), and fungi (Candida albicans ATCC 10231) were used as standard microorganisms. Antimicrobial activity was evaluated in the range of 1280–640–320–160–80–40–20–10 μg/mL concentrations. First of all, strains at – 80 °C were regenerated with NutrienthBroth (Sigma-Aldrich) for bacteria and YeastBroth (Sigma-Aldrich) for fungi. The concentrations of microorganisms were 1 × 108 CFU mL−1 for bacteria and 1 × 106 CFU mL−1 for fungi. The lowest concentration that visibly inhibits the growth of microorganisms in microdilution plates containing various concentrations of nanoparticles was determined as the minimum inhibitory concentration (MIC) according to CLSI standards.
The minimum bactericidal concentration (MBC) is determined by subculture to agar plates or broth without any antibacterial agent at concentrations where there is no visible growth as a result of the MIC test. MBC is the lowest dose of any antimicrobial compound required to kill 100% of a specific bacterium. In this study, MBC value was determined by incubation at 37 °C for 24 h after inoculation on tryptic soy agar medium at the specified concentrations. Sabaraud dextrose agar (SDA) was used for minimum fungicidal concentration (MFC). In addition, percent inhibition was calculated by spectrophotometric analysis of microdilution plates using a microplate reader (ELISA AoMicroplate reader, AzureBiosystems, Inc.).
2.6 Cell Culture
A549 and MCF7 cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM)/ High Glucose supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in an atmosphere of 5% CO2 at 37 °C. For cell culture analysis, stock solutions of gallic acid–CuhNFs and gallic acid–ZnhNFs were prepared and applied at 7 dilutions to cell lines grown in 96-well plates. It was then incubated for 24 h.
2.7 Cytotoxicity Assessment (MTT Assay)
MTT([3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltrazolium bromide]) test was applied to reveal the possible cytotoxic effects of synthesized hNFs and to determine the concentrations that can be used in genotoxicity studies. For this purpose, the A549 cell was seeded at a density of 12 500 cells/well in a 96-well plate, while for MCF-7 cell it was seeded at a density of 5000 cells/well and incubated overnight. Concentrations used in the MTT test were gradually reduced starting from the highest concentration. In this context, gallic acid–CuhNFs and gallic acid–ZnhNFs in the concentration range of 3.9–500 µg/mL were added to the culture medium, and viability was determined using a microplate reader (FilterMax F5, Molecular Devices, USA). The effect of nanoflowers on the viability of A549 and MCF7 cells was evaluated as the percentage of cell viability.
2.8 Statistical Analysis
All data were expressed as the mean values ± SD. Statistical analysis was performed by one-way ANOVA followed by Dunnett’s multiple comparisons test using Graphpad Prism, version 8.0.2 (Graphpad Software Inc., CA, USA). P < 0.05 was considered statistically significant.
3 Results and Discussion
3.1 Characterization of hNFs
3.1.1 SEM Analysis
In the study, CuhNFs and ZnhNFs were successfully synthesized and the morphology of each hNF was monitored with SEM images (Fig. 3).
3.1.2 EDX Analysis
The basic composition and elemental analysis of synthesized gallic acid–CuhNFs and gallic acid–ZnhNFs were determined using energy dispersive X-ray (EDX). The EDX spectrum of synthesized hNFs is given in Fig. 4. The elements Cu, Zn, O, and P found in the spectrum come from the Cu3(PO4)2·3H2O and Zn3(PO4)2·4H2O nanocrystalline.
3.1.3 Elemental Mapping
Also, the elemental mapping of hNFs was performed (Figs. 5 and 6). As seen in Figs. 5 and 6, the elemental mapping analyses confirm the Cu, Zn, O, Cl, and P elements, and the elements are homogeneously distributed inside the gallic acid–CuhNFs and gallic acid–ZnhNFs.
3.1.4 XRD Analysis
The crystal structure of the synthesized gallic acid–CuhNFs and gallic acid–ZnhNFs were elucidated using XRD analysis. As can be seen from the XRD results given in Fig. 7, the XRD peaks assigned for gallic acid–CuhNFs was as follows. XRD: 8.93°, 12.82°, 18.47°, 20.54°, 22.78°, 26.66°, 29.56°, 33.80°, 35.31°, 36.04°, 37.28°, 38.44°, 41.98°, 47.05°, 53.55°, 55.29°, 57.17°, 61.79°, 65.19°, 68.43°, 72.68° in Fig. 7a compared with JCPDS (00–022-0548) and for gallic acid–ZnhNFs, XRD: 9.68°, 16.83°, 17.54°, 18.35°, 19.45°, 20.21°, 22.21°, 22.99°, 24.55°, 25.83°, 26.36°, 27.46°, 28.67°, 31.48°, 33.95°, 34.45°, 35.79°, 37.18°, 38.49°, 39.73°, 41.20°, 41.99°, 43.12°, 45.47°, 46.92°, 50.07°, 52.99°, 54.23°, 55.06°, 56.37°, 57.87°, 59.11°, 60.60°, 61.34°, 66.12°, 69.35°, 71.42°, 75.07°, 77.07°, 82.11° in Fig. 7b compared with JCPDS (01–076-0896).
X-ray diffraction analysis of Cu3(PO4)2·3H2O (red line) nanocrystal of flower-shaped hybrid nanostructures. This analysis complies with the JCPDS (00-022-0548) standard (blue line). X-ray diffraction analysis of Zn3(PO4)2·4H2O (red line) nanocrystal of flower-shaped hybrid nanostructures. This analysis complies with the JCPDS (01-076-0896) standard (blue line).
3.1.5 FTIR Analysis
The chemical structure and formation of gallic acid–CuhNFs and gallic acid–ZnhNFs were investigated using the FT-IR (Perkin Elmer Spectrum 400) spectrum. The FT-IR spectrum revealed characteristic peaks of hNFs (Fig. 8). The data of spectrums of gallic acid–CuhNFs and gallic acid–ZnhNFs were seen as follows. For gallic acid–CuhNFs, FT-IR (cm−1): 3451 (N–H and O–H stretching), 2979 (Ar–H and C–H stretching), 2900 (C–H, stretching), 1647, 1540, 1410, 1150, 1045 (P=O), 1071, 953 (P–O), 799, 617 (O=P=O), 644, 564 (O=P=O), 504, 492, 483, 475, 467. For gallic acid–ZnhNFs, FT-IR (cm−1): 3435 (N–H and O–H stretching), 3270 (Ar–H, stretching), 3184 (Ar–H, stretching), 2964 (C–H, stretching), 2925 (C–H, stretching), 1645, 1560, 1426, 1319, 1244, 1102, 1034, 1020 (P=O), 999 (P=O), 931 (P–O), 708, 635, 628 (O=P=O), 595, 585, 567 (O=P=O), 535, 519, 507, 497, 489, 480, 473, 455.
3.1.6 Determination of Antimicrobial Activity
hNFs were effective against all tested microorganisms (Fig. 9). Interestingly, hNFs showed high inhibition at all concentrations, especially against Gram-positive bacteria S. aureus. Similar to S. aureus, E. coli showed highly inhibited by Cu NPs. P. aeruginosa and C. albicans showed less inhibition, but hNFs also had significant inhibition on these microorganisms when compared with gallic acid concentrations (p < 0.0001). MIC and MBC/MFC values of CuhNFs are given in Tables 1 and 2.
3.1.7 Cytotoxicity Assessment
The effect of GA–CuhNFs and GA–ZnhNFs on the viability of A549 and MCF7 cell lines is shown in Fig. 10. On A549 and MCF7 lines, 8 different concentrations (3.9–7.81–15.625–31.25–62.5–125–250–500 µg/mL) were applied. It was observed that the anticancer activity of the synthesized gallic acid-CuhNFs was quite effective in both the A549 cell line and MCF7 cell line.
4 Conclusion
To summarize, organic–inorganic hNF synthesis was carried out by using gallic acid as an organic fraction and Cu(II), and Zn(II) metal ions as inorganic fractions. The characterization of synthesized gallic acid hNFs was performed using SEM, EDX, XRD, FT-IR, and mapping methods. In addition, the cytotoxic effects of the synthesized gallic acid–CuhNFs and gallic acid–ZnhNFs were investigated on A549 and MCF7 cell lines. It was observed that the anticancer activity of the synthesized gallic acid–CuhNFs was quite effective in both the A549 cell line and the MCF7 cell line. While CuhNFs caused 65% cell death in the A549 cell line, it caused 67% cell death in the MCF7 cell line. ZnhNFs, although not very effective in both cell lines, caused cell death at some concentrations. As the findings indicate, the anticancer activity of hNFs synthesized with Cu was better in both cell lines. When their antimicrobial activities were examined, hNFs showed high inhibition at all concentrations, especially against Gram-positive bacteria S. aureus. Similar to S. aureus, E. coli was also found to be highly inhibited by CuhNFs. P. aeruginosa and C. albicans showed less inhibition, but hNFs showed significant inhibition on these microorganisms compared to gallic acid.
Data Availability
No datasets were generated or analysed during the current study.
References
A.O. Dada, F.A. Adekola, F.E. Dada, A.T. Adelani-Akande, M.O. Bello, C.R. Okonkwo, A.A. Inyinbor, A.P. Oluyori, A. Olayanju, K.O. Ajanaku, C.O. Adetunji, Heliyon 5, 10 (2019). https://doi.org/10.1016/j.heliyon.2019.e02517
B. Mahdavi, S. Saneei, M. Qorbani, M. Zhaleh, A. Zangeneh, M.M. Zangeneh, E. Pirabbasi, N. Abbasi, H. Ghaneialvar, Appl. Organomet. Chem. (2019). https://doi.org/10.1002/aoc.5164
S.A. Khan, F. Noreen, S. Kanwal, A. Iqbal, G. Hussain, Mater. Sci. Eng. C 82, 46–59 (2018). https://doi.org/10.1016/j.msec.2017.08.071
E. Mostafa, M.A.A. Fayed, R.A. Radwan, R.O. Bakr, Colloids Surf. B 182, 110350 (2019). https://doi.org/10.1016/j.colsurfb.2019.110350
Sumbal, A. Nadeem, S. Naz, J.S. Ali, A. Mannan, M. Zia, J. Appl. Biotechnol. Rep. 22, e00338 (2019). https://doi.org/10.1016/j.btre.2019.e00338
J. Cui, S. Jia, Coord. Chem. Rev. 352, 249–263 (2017). https://doi.org/10.1016/J.CCR.2017.09.008
L. Hernandez-Morales, H. Espinoza-Gomez, L.Z. Flores-Lopez, E.L. Sotelo-Barrera, A. Nunez-Rivera, R.D. Cadena-Nava, G. Alonso-Nunez, K.A. Espinoza, Appl. Surf. Sci. 489, 952–961 (2019). https://doi.org/10.1016/j.apsusc.2019.06.031
F. Shao, A.J. Yang, D.M. Yu, J. Wang, X. Gong, H.X. Tian, J. Photochem. Photobiol. B 189, 267–273 (2018). https://doi.org/10.1016/j.jphotobiol.2018.10.014
T.V. Dang, N.S. Heo, H.J. Cho, S.M. Lee, M.Y. Song, H.J. Kim et al., Microchim. Acta 188, 293 (2021). https://doi.org/10.1007/S00604-021-04937-4
Z. Lei, C. Gao, L. Chen, Y. He, W. Ma, Z.J. Lin, Mater. Chem. B 6, 1581–1594 (2018). https://doi.org/10.1039/C7TB03310A
S. Sonia, J.K.H. Linda, K. Ruckmani, M. Sivakumar, Mater. Sci. Eng. C 79, 581–589 (2017). https://doi.org/10.1016/j.msec.2017.05.059
R. Mukhopadhyay, J. Kazi, M.C. Debnath, Biomed. Pharmacother. 97, 1373–1385 (2018). https://doi.org/10.1016/j.biopha.2017.10.167
K. Sriwong, A.A. Koesoema, T. Matsuda, RSC Adv. 10, 30953–30960 (2020). https://doi.org/10.1039/D0RA03160G
S.K.S. Patel, H. Choi, J.-K. Lee, ACS Sustain. Chem. Eng. 7, 13633–13638 (2019). https://doi.org/10.1021/acssuschemeng.9b02583
K. Niska, E. Zielinska, M.W. Radomski, Chem. Biol. Interact. 295, 38–51 (2018). https://doi.org/10.1016/j.cbi.2017.06.018
B.I.A. Kharisov, Review for synthesis of nanoflowers. Rec. Pat. Nanotechnol. 2, 190–200 (2008). https://doi.org/10.2174/18722100878636965
S.G. Yilmaz, A. Demirbas, Z. Karaagac, S. Dadi, C. Celik, S. Yusufbeyoglu, N. Ildiz, A.K. Mandal, B. Cimen, I. Ocsoy, J. Biotechnol. 343, 96–101 (2022). https://doi.org/10.1016/j.jbiotec.2021.11.009
B. Kumar, K. Smita, P. Borovskikh, A. Shchegolkov, A. Debut, L. Cumbal, Inorg. Chem. Commun. 133, 108868 (2021). https://doi.org/10.1016/j.inoche.2021.108868
X. Liang, Y. Liu, K. Wen, W. Jiang, Q. Li, Immobilized enzymes in inorganic hybrid nanoflowers for biocatalytic and biosensing applications. J. Mater. Chem. B 9, 7597–7607 (2021). https://doi.org/10.1039/D1TB01476E
F. Duman, I. Ocsoy, F.O. Kup, Mater. Sci. Eng. C 60, 333–338 (2016). https://doi.org/10.1016/j.msec.2015.11.052
O.C. Güven, M. Kar, F.D. Koca, J. Inorg. Organomet. Polym. Mater. 32, 1026–1032 (2022). https://doi.org/10.1007/s10904-021-02160-5
S. Dayan, C. Altinkaynak, N. Kayaci et al., Appl. Organomet. Chem. 34, e5381 (2020). https://doi.org/10.1002/aoc.538
S.A. Noma, B. Somturk Yilmaz, A. Ulu, N. Ozdemir, B. Ateş, Catal. Lett. 151, 1191–1201 (2021). https://doi.org/10.1007/s10562-020-03362-1
A. Baldemir, N.B. Köse, N. Ildız, S. Ilgün, S. Yusufbeyoğlu, V. Yilmaz, I. Ocsoy, RSC Adv. 7, 44303–44308 (2017). https://doi.org/10.1039/C7RA07618E
J. Ge, J.D. Lei, R.N. Zare, Nat. Nanotechnol. 7, 428–432 (2012). https://doi.org/10.1038/nnano2012.80
M.A. Ebrahimzadeh, A. Naghizadeh, S. Mohammadi-Aghdam et al., J. Photochem. Photobiol. B Biol. 209, 111949 (2020). https://doi.org/10.1016/j.jphotobiol.2020.111949
N. Ildiz, A. Baldemir, C. Altinkaynak, N. Ozdemir, V. Yilmaz, I. Ocsoy, Enzym. Microb. Technol. 102, 60–66 (2017). https://doi.org/10.1016/j.enzmictec.2017.04.003
K.C. Choi, Y.H. Lee, M.G. Jung et al., Mol. Cancer Res. 7, 2011–2021 (2009). https://doi.org/10.1158/1541-7786.MCR-09-0239
J. Daduang, A. Palasap, S. Daduang et al., Asian Pac. J. Cancer Prev. 16, 169–174 (2015). https://doi.org/10.7314/apjcp.2015.16.1.169
R.X. Wang, L.J. Ma, D. Weng et al., Oncol. Rep. 35, 3075–3083 (2016). https://doi.org/10.3892/or.2016.4690
K. Filipiak, M. Hidalgo, J.M. Silvan et al., Food Funct. 5, 381–389 (2014). https://doi.org/10.1039/C3FO60465A
Y. Huang, Y. Chen, Z. Lu, B. Yu, L. Zou, X. Song, H. Han, Q. Jin, J. Ji, Small 19(43), 230578 (2023). https://doi.org/10.1002/smll.202302578
S. Dadi, C. Celik, I. Ocsoy, Sci. Rep. 10, 16765 (2020). https://doi.org/10.1038/s41598-020-73778-7
B. Somturk Yılmaz, H. Bekci, A. Altiparmak, S. Uysal, S. Senkardes, G. Zengin, Process Biochem. 138, 14–22 (2024). https://doi.org/10.1016/j.procbio.2024.01.011
B. Somturk, S. Dayan, N. Ozdemir, N. Kalaycıoğlu Özpozan, Chem. Pap. 76, 4245–4260 (2022). https://doi.org/10.1007/s11696-022-02179-z
B. Somturk, M. Hançer, İ Öcsoy, N. Ozdemir, Dalton Trans. 44(31), 13845–13852 (2015). https://doi.org/10.1039/c5dt01250c
B. Somturk, I. Yilmaz, C. Altinkaynak, A. Karatepe, N. Özdemir, I. Ocsoy, Enzyme Microb. Technol. 86, 134–142 (2016). https://doi.org/10.1016/j.enzmictec.2015.09.005
Acknowledgements
The author would like to thank Erciyes University, Drug Application and Research Center (ERFARMA) for laboratory and device support. We would like to thank the Proofreading & Editing Office of the Dean for Research at Erciyes University for copyediting and proofreading service for this manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the Erciyes University Scientific Research Projects Unit (project coded THD 2023-13214).
Author information
Authors and Affiliations
Contributions
B.S.Y. performed all experiments. B.S.Y. conceived the original idea and designed the project. B.S.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Somtürk Yilmaz, B. Antimicrobial and Anticancer Activity of Gallic Acid–Cu(II) Hybrid Nanoflowers and Gallic Acid–Zn(II) Hybrid Nanoflowers. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03169-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03169-2