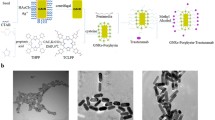
Abstract
Gold nanorods (AuNRs) are powerful photothermal agents (PTAs) in cancer treatment due to their near-infrared laser light absorption ability. However, the cytotoxicity of AuNRs caused by the presence of cationic surfactants often used and their lack of specificity affect their application in photothermal therapy. Thus, we herein developed a bioconjugate obtained from the functionalisation of AuNRs to gelatin (Gel@AuNRs), followed by the conjugation of the as-synthesised material to a breast cancer antibody, trastuzumab (Trast-Gel@AuNRs) to address these issues. The optical and structural characterization of the as-synthesized indicated no significant changes in the optical properties of AuNRs after their functionalisation with gelatin and conjugation with the antibody. The photothermal profiling of the as-synthesised materials showed that AuNRs still have an excellent photothermal conversion efficiency (PCE) after their functionalisation (20%) and their conjugation to an antibody (19%). In addition, the In vitro photothermal depth response showed that Trast-Gel@AuNRs is a promising photothermal agent for HER2-positive breast cancer treatment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer is a heterogeneous disease resulting from breast cell mutation and abnormal growth, forming a mass tissue called a ‘’tumour’’ [1]. It is the most diagnosed cancer in women globally, with over 2.3 million cases occurring yearly and ~ 684,996 deaths globally in 2020 according to WHO [2,3,4]. To date, several conventional treatments have been employed to treat breast cancer. However, numerous limitations have been encountered with the conventional clinical treatments of cancer, and they have fuelled the development of new cancer treatment methods with reduced side effects [5]. Among these various methods, hyperthermia therapy has shown to be a promising alternative for tumour treatment [6]. Different heating sources, such as radiofrequency waves and microwaves, can generate a medium-rise temperature in a particular region to kill cancer cells [3, 7, 8]. However, due to the low absorption of natural tissue absorbents, different photothermal agents such as nanomaterial (carbon-based nanomaterials [9,10,11] or inorganic metal nanomaterials [6, 12, 13]) have been proposed. They are injected into the specific tumour areas to stimulate their photothermal effect or reactive species to kill cancer cells [8]. Another group of nanomaterials known as nanoezymes [14, 15], have been recently developed as a photothermal agent and respond well to NIR light (808 nm). However, the efficiency of these nanozymes for tumours treatment through the photothermal effect still needs improvement [16]. AuNRs on the other hand have gained much attention among the different photo transducer agents due to their interesting optical properties and their ability to absorb near-infrared (NIR) light [13]. Their size, shape, and resonance frequency can easily be tuned through the variation of synthetic parameters hence offering a wide control of their absorption wavelength, which is an advantage for treating deeply embedded tumour [17]. The use of AuNRs as photothermal agents (PTAs) in photothermal therapy (PTT) applications is often limited due to the lack of functionalities, specific delivery and toxicity [18, 19]. Therefore, there have been many reports on the surface modifications of AuNRs with different biocompatible molecules such as natural polymers (gelatin) [12], synthetic polymers (polyethylene glycol, (PEG) [20], and polyvinylpyrrolidone, (PVP) [13]) for added functionalities and to reduce their cytotoxicity [21,22,23]. In our previous work, we have shown that gelatin, a natural polymer, can improve the cytotoxicity and stability of AuNRs [12]. Nevertheless, to meet the rigorous requirements of biological applications such as specificity, these gelatin-stabilized AuNRs need improvement. One way of addressing this challenge is by conjugation to antibodies- which are overexpressed on the tumour surface. Thus, in this work, the gelatin-coated AuNRs (Gel@AuNRs) was conjugated to an antibody (Trastuzumab) and the photothermal response of the resultant material (Tras-Gel@AuNRs) was investigated in a tissue model constructed from Agar. As far as the author knows, such an investigation has not been reported. Trastuzumab is a breast cancer antibody approved by FDA used for the treatment of human epidermal growth factor receptor 2 (HER2) –positive expressing breast cancers [24]. Human breast cancer has been reported to overexpress 20–25% of HER2 [25]. Therefore, conjugating Gel@AuNRs with Trastuzumab is expected to improve Gel@AuNRs breast cancer treatment efficiency. Hoever, whether this conjugation would hinder AuNRs’ photothermal properties or not during irradiation in the tissue still needs to be investigated. In this study, the photothermal properties of Gel@AuNRs conjugated with trastuzumab and its response after being injected at a 15 mm depth using an Agar model as a bio-simulation material for tissue were investigated. Agar was chosen because it is a hydrogel with absorption and scattering features similar to soft tissue. It is often used to evaluate the heat produced following its laser beam irradiation and the heat distribution [26, 27]. Moreover, the presence of hydrophilic groups in Agar gels allows them to retain water in their structures and readily construct desired shapes due to their ability to form semi-solids [28, 29]. The results showed that functionalising AuNRs with biomolecules such as gelatin does not affect the photo response in a tissue, even when an antibody is attached and the as-synthesised Tras-Gel@AuNRs have the potential to be used as PTAs for breast cancer treatment.
2 Methodology
2.1 Materials
Hydrogen tetrachloroaurate hydrate (HAuCl4, 99.9%), Cetyltrimethylammonium bromide (CTAB, ≥ 99%), Sodium borohydride (NaBH4, 99%), Silver nitrate (AgNO3, 99%),, Hydroquinone (HQ, 99%), N-hydroxysuccinimide (NHS), Sodium hydroxide (NaOH), Hydrochloric acid, HCl (ACS reagent 37 wt%) were purchased from Sigma-Aldrich. Gelatin was obtained from Fluka. Ascorbic acid was obtained from the Platinum line. Ethyl (dimethylamino propyl) carbodiimide Hydrochloride, EDC-HCl, was purchased from Roth. The agar was purchased from Japanese Ina agar F. The Ultrasonic Gel was purchased from Nihon Kohden Co (Japan). The red ink (food dye) was purchased from Kyoritsu Foods Co. (Japan). No further purification was required for all the chemicals prior to their use. Fresh solutions besides the HAuCl4, HCl, and NaOH were used throughout the work. All glassware used in the experiments was rigorously washed with aqua regia, soap, and deionised water and dried prior to every use.
2.2 Experimental Details
2.2.1 Synthesis of Gold Nanorods
The synthesis of AuNRs was carried out following Chang et al. [30] methods with slight modifications. The seed solution was prepared by adding HAuCl4·xH2O (0.01 M, 0.50 mL) to CTAB (0.1 M, 9.5 ml), and the mixture was stirred for 15 min at 450 rpm. Then 0.46 mL of a freshly prepared ice-cold solution of 0.01 M NaBH4 in NaOH was added to the mixture under continuous stirring for 2 min. After 2 min of stirring, the solution was kept unstirred at ambient temperature for more than 30 min. The growth solution of AuNRs was synthesised by sequentially adding HAuCl4·xH2O (0.5 mL, 0.01 M), AgNO3 (0.01 mL, 0.1 M); HCl (200 µL, 1.0 M) to 9 mL of CTAB, and the solutions were gently stirred. Then, AA (80 µL, 0.1 M) was added, and the solution turned colourless from yellow. Finally, seed solution (1 mL) was added, and the solution was kept unstirred for 16–20 h at room temperature and analysed the following day using the Ultraviolet-visible-near infrared spectroscopy (UV-Vis-NIR).
2.2.2 Functionalisation of Gold Nanorods Using Gelatin
Gelatin-stabilised AuNRs (Gel@AuNRs) were synthesised as reported by Oladipo et al. [12] with slight modifications. Briefly, gelatin solution (dissolved in warm water, 10 mg/mL) was mixed with AuNRs. The 1:2 nanorods to gelatin volume ratio was used (2 mL of gelatin with 4 mL of AuNRs).
2.2.3 Conjugation of Gelatin-Stabilised Gold Nanorods to Trastuzumab
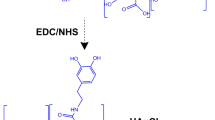
Conjugation of the antibody trastuzumab to Gel@AuNRs was done according to the method reported by Hamaly et al. [31] with slight modifications following scheme 1. Typically, fresh aqueous solutions of EDC-HCl (100 µL of 0.05 M) and NHS (100 µL of 0.05 M) were prepared and sequentially added to 1 mL of Gel@AuNRs suspension at room temperature and left for 20 min to activate the terminal carboxylic acid groups on Gel@AuNRs. Trastuzumab solution (10 µL of 10 mg/mL) was then added to the Gel@AuNRs suspension, and the mixture was left overnight at 4 °C without stirring. The product was characterised the following day.
2.2.4 Characterization
The absorption properties of the samples were obtained a using Ultra violet-Visible near infrared (UV-vis-NIR) absorption with a JASCO V-770 NIR spectrophotometer; Dynamic light scattering spectroscopy (DLS) was done to evaluate the surface charge of the samples using a Microtrac Nanotrac Wave II; a Perkin Elmer Spectrum Two spectrophotometer was used to obtain all the Fourier transform infrared (FTIR) spectra, and the morphology of the samples was obtained using transmission electron microscopy (TEM) with a JEOL 2100 F HRTEM at an accelerating voltage of 200KV. The purification of the materials was done via centrifugation using a Sigma 3–30 KS and a Pro-Analytical Centurion scientific.
2.2.5 Photothermal Profiling
Photothermal analysis was done by adding AuNRs, Gel@AuNRs, or Tras-Gel@AuNRs sample into a spectroscopic cuvette and irradiating it with an 808 nm laser (OSTECH electro-optical instrument, 1.5 W). The changing temperature was measured using an RS-PRO-1384 thermocouple for 10 min and a FLIR E4 thermal camera.
The temperature change was obtained using the equation below:
Where\(\varDelta T\) is the change in temperature\(, {T}_{i}\) is the initial temperature at 0 min and \({T}_{f}\): is the final temperature at different time intervals.
The photothermal conversion efficiency (PCE) was evaluated according to Lebepe et al. [6] with slight modifications. Briefly, 2 mL of each sample was irradiated with an 808 nm laser with a power density of 1.5 W·for 10 min, and then the laser was turned off. The cooling temperature of the dispersions was recorded every 30 s using a thermocouple. The PCE was calculated following Liu et al.’s calculation with modifications [32].
2.2.6 In vitro Photothermal Depth Response Profiling
The photothermal depth response profiling was done following Obonai et al. [27] procedure with slight modifications. Briefly, 0.3192 g of each sample (AuNRs, Gel@AuNRs, or Tras-Gel@AuNRs) was mixed with the red ink (0.0065 g) and the ultrasonic gel (0.3192 g), separately. The mass of all these samples was measured using an electric balance (METTLER TOLEDO AE 163, Laboratory and weighing Technologies, Switzerland). The mixture (200 µL) was slowly injected into the agar at 15 mm depth with the aid of the syringe and an injector, and the agar was irradiated for 5 min using an 808 nm laser system. The laser apparatus was composed of a power supply unit (Changchun new industries Optoelectronics Tech Model No. PSU-H-FDA, China), an arbitrary wave function generator (GW INSTEK, Model No. AFG-2125, Arbitrary / Direct Digital Synthesis (DDS), 1 Channel, 25 MHz, AFG-2100 Series, USA) and an infrared diode laser (Changchun new industries Optoelectronics Tech Model No. MDL-N-808/7000 ~ 10,000 mW, China).
3 Results and Discussion
3.1 Synthesis of Gold Nanorods and Gelatin-Stabilised Gold Nanorods
The synthesis of AuNRs was done using a seed-mediated method reported by Chang et al. with slight modifications [30]. In this method, the growth of AuNRs was done at low pHs to promote the formation of mini AuNRs. As previously reported in our work, Gel@AuNRs were further synthesised by overcoating the existing AuNRs with a gelatin layer [12]. The as-synthesised materials were characterised using UV-vis-NIR, FTIR, DLS, and TEM. The absorption spectra of AuNRs and Gel@AuNRs (Fig. 1.a) show that both materials display transverse peaks at around 519 nm while the longitudinal peak of Gel@AuNRs is slightly red-shifted. The absence of the longitudinal peak broadening also indicates a high shape purity and monodispersity. The FTIR spectra of AuNRs (Fig. 1. b) confirms the presence of strong CTAB -CH stretching peaks around 2925 cm− 1 and 2840 cm− 1 and free O-H around 3395 cm− 1 [12] The FTIR analysis of Gel@AuNRs further confirmed the effective coating of gelatin to AuNRs by the presence of free O-H at around 3283 cm− 1, a C = O stretching vibrations at around 1633 cm− 1, and a C-N stretching vibrations located at around 1532 cm− 1. Additionally, the -CH2 stretching peaks from CTAB were still maintained with a lower intensity [12, 33]. The DLS results (Fig. 1. c and d) indicate that AuNRs display distinct peaks while coating with gelatin increased the broadness of these peaks as well as their hydrodynamic length from 27.00 to 62.90 nm respectively. The presence of gelatin increases the material viscosity, hence Gel@AuNRs tend to have a broader peak and a bigger size when analysed using DLS methods [34]. The zeta potential of both materials was + 39.43 mV and + 21.00 mV respectively. The polarity change also confirms the change in the AuNRs surface due to gelatin coating. The TEM micrographs (Fig. 1.e and f) show that both AuNRs and Gel@AuNRs are rod-like, crystalline and monodispersed with an average length of 28.60 ± 9.59 and 28.91 ± 7.85 nm, respectively.
3.2 Conjugation of Gelatin-Stabilised Gold Nanorods to Trastuzumab
The conjugation of Trastuzumab to Gel@AuNRs (Trast-Gel@AuNRs) was obtained via covalent bonding of the primary amine group of the antibody and the carboxylic group of gelatin on the surface of AuNRs using EDC-HCl and sulpho-NHS as the intermediates cross-linkers. The conjugation process was initiated by adding EDC, which activated the carboxylic acid terminal of Gel@AuNRs from gelatin to give an unstable ester bond. The addition of sulpho-NHS replaced the unstable ester bond of EDC-HCl and gelatin to form an amine-reactive NHS-ester, which acted as an intermediate for conjugating Gel@AuNRs with Trastuzumab via the covalent bond of the amide and carboxylic group (Scheme 1) [31, 35].
The conjugation was evaluated using UV-Vis-NIR, FTIR and DLS. The absorption spectra of Trast-Gel@AuNRs (Fig. 2.a.) revealed an insignificant red shift in the LSPR peak compared to Gel@AuNRs. The TSPR absorbance increased compared to the initial Gel@AuNRs spectra. This might be due to the change in the optical nature of Gel@AuNRs because of the antibody absorbance in the higher energy wavelength. The efficient conjugation of Gel@AuNRs to Trastuzumab was confirmed by FTIR (Fig. 2.b). The FTIR spectrum of Trast-Gel@AuNRs shows an N-H coupled with OH attributed to the presence of gelatin and an amide stretch located in the region between 1600 and 1800 cm− 1. The less intense C-H stretching bands from methyl and methylene groups from both Gel@AuNRs and Trast-Gel@AuNRs are seen at 2925 and 2840 cm− 1 [36, 37] respectively. The shift of the peaks around 1200 and 500 cm− 1 might be due to the interaction between Gel@AuNRs and Trastuzumab.
The DLS histogram of Gel@AuNRs displayed two peaks which were converted into one broad and sharper peak after the formation of Trast-Gel@AuNRs. This confirms the change in the diffusion coefficient of Gel@AuNRs after conjugation as shown in Fig. 2c and d [38, 39]. The disappearance of the small size peak which is typical of the rotational movement of the nanorods is an indication of aggregate fornation. The interaction of Gel@AuNRs with the antibody might have induced the formation of an aggregate by disrupting the CTAB protective bilayer. The zeta potential of Gel@AuNRs changed from + 21 mV to + 7.7 mV after conjugation due to the anionic potential of trastuzumab [40] and thus, indicates the successful conjugation of trastuzumab on the surface of Gel@AuNRs.
3.3 Photothermal Profiling of Gold Nanorods, Gelatin Stabilised Gold Nanorods and Trast-Gel@AuNRs
AuNRs are known as excellent PTAs, and it is, therefore, important to investigate the effect of bioconjugation on its photothermal properties. The photothermal profiling of AuNRs, Gel@AuNRs and Trast-Gel@AuNRs was evaluated by irradiating them with an 808 nm laser (1.5 W) for 10 min. The temperature changes were monitored using the thermocouple and thermal camera. The temperatures from the thermocouple obtained after 10 min of irradiation were 57.8, 58.9 and 56.0 ℃ representing a temperature change of 30.8, 31.9, and 29.0 ℃ for AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs, respectively (Fig. 3.a) with the initial temperature of 27 ± 3.0 ℃. The conjugation showed a decrease in temperature change compared to bare AuNRs and Gel@AuNRs. This could be attributed to the interference of the light by the antibody. However, the temperature was sufficient to destroy the cancer cells, as it has been reported that a temperature of 45 ℃ is appropriate to destroy the cancer cells [6, 41]. The thermal camera was used to capture photographic thermal images from the beginning to the end. The results show temperature changes of 32.7, 28.7 and 28.5 ℃ for AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs, respectively (Fig. 3.b), which further confirms the thermocouple temperature changes obtained. The photothermal conversion efficiency (PCE) of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs was calculated to be 27.66, 20.00 and 19.00%. usingEqn. 2 [32].
Where hS is the heat transfer coefficient and the surface area of the container with the values of 9.52, 7.11, and 7.03 mW/℃ for AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs, respectively. Tmax is the maximum steady temperature, Tsurr is the environmental temperature, I is the laser power at 1.5 W, A808 is the absorbance of the AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs at 808 nm which are 0.83, 1.03 and 1.07 a.u respectively while QDis (9.31 × 10− 3 W/℃) is the heat dissipated from the light absorbed by the solvent (water) and the container. Fig. 3c-f shows the linear plot of the cooling temperature after reaching the maximum steady temperature (Tmax). The Tmax of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs recorded from the thermocouple was 65.1, 66.5, and 65.3 ℃ respectively with the environmental temperature (TSurr) of 27 ± 3.0 ℃. The results showed that functionalisation and conjugation do not have any significant impact on the AuNRs’ photothermal properties. The absorption spectra of the AuNRs, Gel@AuNRs and Trast-Gel@AuNRs after 60 min irradiation is shown in Fig. S1. The conjugate showed a minimal blue shift in the LSPR peak after irradiation which shows that it posses good photothermal stability. The photothermal property of the conjugate compared to different reported PTT nanomaterials (Table 1) shows that it is an efficient photothermal agent.
(a) Thermocouple temperature changes and (b) IR thermal camera images of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs irradiated for 10 min with 808 nm Laser. (c) The temperature changes during the irradiation of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs and cooling down after switching off the laser. The temperature profile during the cooling period of (d) AuNRs, (e) Gel@AuNRs, and (f) Trast-Gel@AuNRs
3.4 In vitro Photothermal Depth Response of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs
Cancer phototherapy often involve the treatment of deep-seated tumours [8]. Therefore, it is essential to investigate the PTT depth response profiling of PTAs and evaluate their temperature production because biological tissues have an intense light scattering ability which affects the penetration depth of the NIR laser light and the efficiency of PTAs [33, 47]. In this work, we investigated the effect of the antibody and gelatin on the AuNRs depth response during the irradiation in the tissue simulation. For this purpose, an Agar was used to simulate biological tissue to assess the PTT depth profiling of AuNRs, Gel@AuNRs, and Trast-Gel@AuNRs (Fig. 4).
The as-synthesised materials were mixed with red ink (for clear visualisation) and a gel (gelsonic, to increase the viscosity of each sample) and slowly injected into an agar which has settled into a gel-like in a specially made container (Fig. 4 photograhic image). It has been reported that the distance between the nipple and breast tumour can vary [48, 49] thus, thermocouple K-type wires with different channels were embedded to varying depths of 2, 5, 10, 15, and 20 mm, as shown in Fig. 4. In the absence of high-viscosity gel, AuNRs did not settle in the gel. The temperature changes during the irradiation with 808 nm Laser (3.43 W) were measured at different depths 2, 5, 10, 15, and 20 mm using a thermocouple with a time interval of 1 min for the duration of 5 min. The samples (AuNRs, Gel@AuNRs, or Trast-Gel@AuNRs) were injected at a depth of 15 mm in a separate experiment. The control for this experiment was the laser irradiation alone on the Agar gel, which produced temperature change of 25.30, 17.20, 7.60 5.80, and 4.5 ℃ at 2, 5, 10 15, and 20 mm, respectively, which is not sufficient enough to destroy cancerous cells [50]. The blank agar’s highest temperature produced was at 2 mm depth, the closest point to the surface (Fig. 5.a.).The heat production decreases with increasing depth, confirming the low light penetration similar to the natural tissue. The depths of 15 and 20 mm were the least penetrated by the irradiated NIR light when blank agar was irradiated. Thus in our designed experiment, the three materials were injected at 15 mm to assess their impact on heat production. The temperature changes recorded for all three materials were 34.25, 35.97, and 38.95 ℃ for AuNRs, Gel@AuNRs, or Trast-Gel@AuNRs, respectively (Fig. 5. b-d), which was higher than that of the agar gel alone at the same point (15 mm). The temperature changes increased with time, similar to the photothermal profiling obtained after irradiating the three materials in an aqueous solution. The results indicate that Trast-Gel@AuNRs can efficiently produce heat in 15 mm tissue depth if used for breast cancer. Furthermore, we observed that for all the three materials, the heat production did not only improve at 15 mm depth when the NIR light was irradiated but other depths were alos affected positively as shown in Table 1. The temperature rise recorded at 2, 5, 10, and 20 mm were 34.10, 31.60, 18.95, 20.52 ℃ for AuNRs; 35.90, 29.82, 32.57, and 11.90 ℃ for Gel@AuNRs; and 35.30, 24.57, 23.75, and 11.00 ℃ for Trast-Gel@AuNRs.
The temperature change recorded at other depths was strongly influenced by the dispersability of the AuNRs particles. In the case of AuNRs there is a significant difference between the temperature change recorded at 15 mm (34.25℃) and the neighbouring depth, 10 mm (18.95℃). This can be explained by the presence of a higher density of AuNRs particles at 15 mm and they might have spread toward 20 mm where a temperature change of 20.52℃ was recorded. In the case of Gel@AuNRs and Trast-Gel@AuNRs, it could be seen that after the injection of the material into the agar, it did not only remain at 15 mm (with temperature changes of 35.97℃ and 38.95℃) but also dispersed toward 10 mm justifying the elevated temperatures recorded at this depth (32.57℃ and 23.75℃). The amount of AuNRs is believed to influence the depth-related temperature rise in photothermal therapy [51].
4 Conclusion
Mini-AuNRs were synthesised via a seed-mediated method and further functionalised with gelatin via an overcoating process. The results indicated that there was a slight red-shift of AuNRs’ LSPR after the coating process, while FTIR and DLS confirmed the efficiency of the coating process with the presence of gelatin’s C = O and C-N functional groups and the decrease in the zeta potential of AuNRs. Furthermore, a bioconjugate made of gelatin-functionalised AuNRs and trastuzumab (Trast-Gel@AuNRs) was synthesised using conventional EDC/NHS coupling methods. The bioconjugate was formed via covalent bonding between the gelatin carboxylic acid group and the amine group in the antibody. The absorption spectrum of Trast-Gel@AuNRs showed a slight red-shifted compared to Gel@AuNRs. FTIR of Gel@AuNRs presented an amide stretch from the antibody and the change of Gel@AuNRs surface charge due to the anionic antibody attached. PTT profiling of Gel@AuNRs and Trast-Gel@AuNRs did not show any significant reduction in AuNRs photothermal response. An in vitro photothermal analysis using an agar gel at different depths shows a similar laser response after irradiation with a maximum result at 15 mm. The results indicate that Trast-Gel@AuNRs can efficiently produce enough heat at 15 mm tissue depth with high specificity when used for breast cancer therapy.
Data Availability
Not Applicable.
References
N. Harbeck et al., Breast cancer. Nat. Rev. Dis. Primers. 5(1) (Dec. 2019). https://doi.org/10.1038/S41572-019-0111-2
S. Sarangi, P. Sopory, S. Pattnaik, K. Reeta, Antibody–drug conjugates, cancer immunotherapy, and metronomic chemotherapy as novel approaches in cancer management. Indian J. Pharmacol. (2020). https://doi.org/10.4103/ijp.ijp_475_18
A.C.V. Doughty, A.R. Hoover, E. Layton, C.K. Murray, E.W. Howard, W.R. Chen, Nanomaterial applications in photothermal therapy for cancer. Materials. 12(5) (2019). https://doi.org/10.3390/ma12050779
S. Akkın, G. Varan, E. Bilensoy, A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules. (2021). https://doi.org/10.3390/molecules26113382
H. Maeda, M. Khatami, Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl Med. (2018). https://doi.org/10.1186/s40169-018-0185-6
T.C. Lebepe, O.S. Oluwafemi, Photothermal Conversion profiling of large-scaled synthesized Gold Nanorods using binary surfactant with hydroquinone as a reducing Agent. Nanomaterials. 12(10) (2022). https://doi.org/10.3390/nano12101723
M.R.K. Ali, Y. Wu, M.A. El-Sayed, Gold-nanoparticle-assisted Plasmonic Photothermal therapy advances toward clinical application. J. Phys. Chem. C (2019). https://doi.org/10.1021/acs.jpcc.9b01961
Y. Liu, P. Bhattarai, Z. Dai, X. Chen, Photothermal therapy and photoacoustic imaging: Via nanotheranostics in fighting cancer. Chem. Soc. Rev. (2019). https://doi.org/10.1039/c8cs00618k
P. Sundaram, H. Abrahamse, Phototherapy Combined with Carbon nanomaterials (1D and 2D) and their applications in Cancer Therapy. Materials. 13(21) (2020). https://doi.org/10.3390/ma13214830
X. Wang, L. Zhu, Z. Gu, L. Dai, Carbon nanomaterials for phototherapy, 11, 22, pp. 4955–4976, 2022, doi: https://doi.org/10.1515/nanoph-2022-0574
B. Li, S. Zhao, L. Huang, Q. Wang, J. Xiao, M. Lan, Recent advances and prospects of carbon dots in phototherapy. Chem. Eng. J. 408, 127245 (2021). https://doi.org/10.1016/j.cej.2020.127245
A. Oladipo et al., Synthesis of nir-ii absorbing gelatin stabilized gold nanorods and its photothermal therapy application against fibroblast histiocytoma cells. Pharmaceuticals. 14(11) (2021). https://doi.org/10.3390/ph14111137
K.K. Khoza, T.C. Lebepe, G. Mbaz, O.S. Oluwafemi, The Effect of Different Stabilisers on Stability and Photothermal Profiling of Gold Nanorods, J Inorg Organomet Polym Mater, 2023, https://doi.org/10.1007/s10904-023-02691-z
M. Tang et al., Dual active nanozyme-loaded MXene enables hyperthermia-enhanced tumor nanocatalytic therapy. Chem. Eng. J. 449 (Dec. 2022). https://doi.org/10.1016/j.cej.2022.137847
J. Liu et al., Polyoxometalate nanomaterials for enhanced reactive oxygen species theranostics, Coordination Chemistry Reviews, vol. 472. Elsevier B.V., Dec. 01, 2022. https://doi.org/10.1016/j.ccr.2022.214785
J. Li et al., Co-based Nanozymatic Profiling: Advances Spanning Chemistry, Biomedical, and Environmental Sciences, Advanced Materials, vol. 36, no. 8. John Wiley and Sons Inc, Feb. 22, 2024. https://doi.org/10.1002/adma.202307337
T.C. Lebepe, S. Parani, O.S. Oluwafemi, Graphene oxide-coated gold nanorods: synthesis and applications. Nanomaterials. (2020). https://doi.org/10.3390/nano10112149
C. Gui, D.X. Cui, Functionalized gold nanorods for tumor imaging and targeted therapy. Cancer Biol. Med. (2012). https://doi.org/10.7497/j.issn.2095-3941.2012.04.002
F. Scaletti, C.S. Kim, L. Messori, V.M. Rotello, Rapid purification of gold nanorods for biomedical applications. MethodsX. 1, e118–e123 (2014). https://doi.org/10.1016/J.MEX.2014.07.007
R. Marasini, A. Pitchaimani, T.D.T. Nguyen, J. Comer, S. Aryal, The influence of polyethylene glycol passivation on the surface plasmon resonance induced photothermal properties of gold nanorods. Nanoscale. 10(28), 13684–13693 (2018). https://doi.org/10.1039/C8NR03026J
W.M. Park, B.G. Choi, Y.S. Huh, W.H. Hong, S.Y. Lee, T.J. Park, Facile functionalization of colloidal gold nanorods by the specific binding of an engineered protein that is preferred over CTAB bilayers. Chempluschem. 78(1), 48–51 (Jan. 2013). https://doi.org/10.1002/CPLU.201200239
W.C. Wu, J.B. Tracy, Large-scale silica overcoating of gold nanorods with tunable shell thicknesses, Chemistry of Materials, vol. 27, no. 8, pp. 2888–2894, Apr. 2015, https://doi.org/10.1021/cm504764v
Y. Tao et al., Dialysis assisted ligand exchange on gold nanorods: amplification of the performance of a lateral flow immunoassay for E. Coli O157:H7. Microchim. Acta. 185(7) (2018). https://doi.org/10.1007/s00604-018-2897-0
A.H. Boekhout, J.H. Beijnen, J.H.M. Schellens, Trastuzumab, Oncologist, vol. 16, no. 6, pp. 800–810, Jun. 2011, https://doi.org/10.1634/THEONCOLOGIST.2010-0035
N. Ismaili, R. Belbaraka, A. Elomrani, M. Khouchani, A. Tahri, [Recent advances in targeted therapies in the treatment of HER2-positive metastatic breast cancer], Presse Med, vol. 42, no. 11, pp. 1461–1468, Nov. 2013, https://doi.org/10.1016/J.LPM.2013.01.066
K. Pal, A.K. Banthia, D.K. Majumdar, Polymeric hydrogels: characterization and biomedical applications. Des. Monomers Polym. 12(3), 197–220 (May 2009). https://doi.org/10.1163/156855509X436030
A. Obonai, T. Kogawa, Y. Kanda, O.S. Oluwafemi, T. Kodama, A. Komiya, Temperature distribution analysis using a combination of near-infrared laser, gold nanorods, and surface cooling equipment temperature distribution study. Appl. Therm. Eng. 229, 120579 (2023). https://doi.org/10.1016/j.applthermaleng.2023.120579
M. Milanič, B. Majaron, J.S. Nelson, Pulsed photothermal temperature profiling of agar tissue phantoms, Lasers Med Sci, vol. 22, no. 4, pp. 279–284, Nov. 2007, https://doi.org/10.1007/S10103-007-0455-9
Open J Radiol, vol. 04, no. 01, pp. 44–52, 2014, doi: 10.4236/OJRAD.2014.41006
H.H. Chang, C.J. Murphy, Mini gold nanorods with tunable Plasmonic peaks beyond 1000 nm. Chem. Mater. (2018). https://doi.org/10.1021/acs.chemmater.7b05310
M.A. Hamaly, S.R. Abulateefeh, K.M. Al-Qaoud, A.M. Alkilany, Freeze-drying of monoclonal antibody-conjugated gold nanorods: colloidal stability and biological activity. Int. J. Pharm. 550, 1–2 (2018). https://doi.org/10.1016/j.ijpharm.2018.08.045
X. Liu et al., Facile synthesis of biocompatible cysteine-coated CuS nanoparticles with high photothermal conversion efficiency for cancer therapy. Dalton Trans. 43, 11709–11715 (2014). https://doi.org/10.1039/c4dt00424h
M.F. Tsai et al., Jun., Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy, ACS Nano, vol. 7, no. 6, pp. 5330–5342, 2013, https://doi.org/10.1021/NN401187C
S.M. Ahsan, C.M. Rao, The role of surface charge in the desolvation process of gelatin: implications in nanoparticle synthesis and modulation of drug release, Int J Nanomedicine, vol. 12, pp. 795–808, Jan. 2017, https://doi.org/10.2147/IJN.S124938
M.H. Jazayeri, H. Amani, A.A. Pourfatollah, H. Pazoki-Toroudi, B. Sedighimoghaddam, Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sensing Res. 9, 17–22 (2016). The Authors10.1016/j.sbsr.2016.04.002
M.V. Natu, J.P. Sardinha, I.J. Correia, M.H. Gil, Controlled release gelatin hydrogels and lyophilisates with potential application as ocular inserts, Biomedical Materials, vol. 2, no. 4, pp. 241–249, Dec. 2007, https://doi.org/10.1088/1748-6041/2/4/006
P.F.S. Jerry, R. Mohrig, C.N. Hammond, Infrared Spectroscopy, in Techniques in Organic Chemistry, New York: W. H. Freeman, 2010, 2010, pp. 1–463
H. Liu, N. Pierre-Pierre, Q. Huo, Dynamic light scattering for gold nanorod size characterization and study of nanorod-protein interactions, Gold Bull, vol. 45, no. 4, pp. 187–195, Dec. 2012, https://doi.org/10.1007/S13404-012-0067-4
D. Lehner, H. Lindner, O. Glatter, Determination of the translational and rotational diffusion coefficients of rodlike particles using depolarized dynamic light scattering, Langmuir, vol. 16, no. 4, pp. 1689–1695, Feb. 2000, https://doi.org/10.1021/LA9910273
F.S. Aleanizy et al., Trastuzumab targeted neratinib loaded poly-amidoamine dendrimer nanocapsules for breast cancer therapy, Int J Nanomedicine, vol. 15, no. August, pp. 5433–5443, 2020, https://doi.org/10.2147/IJN.S256898
X. Zhu et al., Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 7(1), 10437 (2016). https://doi.org/10.1038/ncomms10437
Y. Liu, D. Zhu, Y. Hu, M.T. Swihart, W. Wei, Controlled Synthesis of Cu2–xSe Nanoparticles as Near-Infrared Photothermal Agents and Irradiation Wavelength Dependence of Their Photothermal Conversion Efficiency, Langmuir, vol. 34, no. 46, pp. 13905–13909, Nov. 2018, https://doi.org/10.1021/acs.langmuir.8b02133
D. Maziukiewicz, B.F. Grzéskowiak, E. Coy, S. Jurga, R. Mrówczýnski, NDs@PDA@ICG conjugates for photothermal therapy of glioblastoma multiforme. Biomimetics. 4(1) (Mar. 2019). https://doi.org/10.3390/biomimetics4010003
B. Wang et al., Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials, 35, 6, pp. 1954–1966, 2014, https://doi.org/10.1016/j.biomaterials.2013.11.066
P. Manivasagan et al., A multifunctional near-infrared laser-triggered drug delivery system using folic acid conjugated chitosan oligosaccharide encapsulated gold nanorods for targeted chemo-photothermal therapy. J. Mater. Chem. B 7(24), 3811–3825 (2019). https://doi.org/10.1039/C8TB02823K
Q. Pei et al., Albumin-bound paclitaxel dimeric prodrug nanoparticles with tumor redox heterogeneity-triggered drug release for synergistic photothermal/chemotherapy. Nano Res. 12(4), 877–887 (2019). https://doi.org/10.1007/s12274-019-2318-7
T. Sugiura et al., Dec., Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light with controlled surface cooling, Nano Res, vol. 8, no. 12, pp. 3842–3852, 2015, https://doi.org/10.1007/S12274-015-0884-X
Q. Yang, J. Yang, L. Xu, C. Zhou, Q. Lv, Distance between tumor and nipple as a prognostic factor in breast cancers: opposite effects in young and old patients. Medicine. 99(32), e21461 (2020). https://doi.org/10.1097/MD.0000000000021461
G. Bentel, L.B. Marks, P. Hardenbergh, L. Prosnitz, Variability of the location of internal mammary vessels and glandular breast tissue in breast cancer patients undergoing routine CT-based treatment planning. Int. J. Radiation Oncology*Biology*Physics. 44(5), 1017–1025 (1999). https://doi.org/10.1016/S0360-3016(99)00123-6
Y. Zhang et al., Recent progress on NIR-II Photothermal Therapy. Front. Chem. 9 (Jul. 2021). https://doi.org/10.3389/FCHEM.2021.728066
B. Jang, K. Sun-young, Y. Choi, Effects of Gold Nanorod Concentration on the Depth-Related Temperature Increase During Hyperthermic Ablation, Small, vol. 7, pp. 265–270, Jan. 2011, https://doi.org/10.1002/smll.201001532
Acknowledgements
The authors would like to thank the National Research Foundation (NRF), South Africa, under the South Africa/Japan bilateral program (Grant number 131590), Equipment-Related Travel and Training grants (Grant number 118666), Competitive Program for Rated Researchers (Grant numbers 129290). University of Johannesburg, South Africa, the research committee (URC), and the Faculty of Science Research Committee (FRC) for financial support.
Funding
This work was supported financially by National Research Foundation (NRF), South Africa, under the South Africa/Japan bilateral program (Grant number 131590 and 151337), Equipment-Related Travel and Training grants (Grant number 118666), Competitive Program for Rated Researchers (Grant numbers 129290). University of Johannesburg, South Africa, the research committee (URC), and the Faculty of Science Research Committee (FRC) for financial support.
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Contributions
G.I.M.M: Investigation, Formal analysis, Writing - original draft and editing. T.C.L. Methodology, Validation, Writing - review & editing. Rodney Maluleke: Formal analysis. A.O.: Methodology and formal analysis. N.M.: formal analysis. O.A.A.: review and editing. R.K.: review and editing, T.K.: formal analysis. A.K.: Project administration, funding aqcuisition & formal analysis. O.S.O.: Conceptualization, Methodology, Resources, Validation, Writing - review & editing, Supervision, Project administration, Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Conflict of Interest
The authors have no known personal or financial competing interests that could influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mwad Mbaz, G., Calvin Lebepe, T., Maluleke, R. et al. Photothermal Depth Profiling of Gelatin-Stabilised Gold Nanorods-Trastuzumab Conjugate as a Potential Breast Cancer Photothermal Agent. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03151-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03151-y