Abstract
In this study, aqueous Cystoseira crinite extract was used to successfully biosynthesize ZnO and C-doped ZnO samples. X-ray diffraction (XRD), scanning electron microscope (SEM), energy dispersive X-ray (EDX), FTIR, and UV–vis techniques were used to characterize the biosynthesized samples. XRD technique confirmed the crystalline structure of ZnO and C-doped ZnO. EDX confirmed the formation of C-doped ZnO. SEM analysis revealed the spherical shape of the samples with agglomeration. FTIR spectra showed that phenolic compounds and protein molecules are present in the Cystoseira crinite extract. The photocatalytic activity of the prepared photocatalysts was investigated using crystal violet (CV) as a model of the cationic dyes and methyl orange (MO) as a model of the anionic dyes. 5%C-doped ZnO calcined at 500 °C showed an optimum photocatalytic efficiency of 92.7% and 87.8% for CV and MO dyes, respectively. The improved photocatalytic activity could be attributed to the narrower optical band gap of C doped ZnO than pure ZnO. The photocatalytic activity of CV and MO dyes follows the first-order kinetics with high correlation coefficient values.
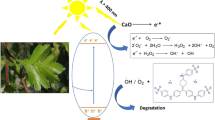
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
However the value of organic dyes in the fabric manufacturing, they are one of the water contaminants, carcinogenic and dangerous [1, 2]. They are harmful to aquatic organisms and prevent light energy from entering the water (causing slow photosynthesis). Recently, removal of dyes from water is considered a challenge. Methods for reusing dye effluent have recently received concern due to the possibility that, in the absence of a reliable solution, clean and drinkable water may start to diminish in a short time [3, 4].
Nowadays, multiple research articles have established various dye removal techniques that claim to be successful. The ideal dye removal method shows a large dye removal percent in a short period without producing secondary pollutants [5]. Various techniques, such as adsorption, membrane filtration, photo-oxidation, chemical oxidation, advanced oxidation processes (AOP), and biodegradation have been improved to decrease the dye percent in water sources [6, 7].
Heterogeneous photocatalysis is considered one of the AOPs for eliminating dyes from industrial wastewater. Several research groups have investigated different pure metal oxides or supported metal oxides catalysts for the photocatalysis process [8, 9]. The photocatalysis technique works by exposing the catalyst to light energy greater than or equal to the photocatalyst's energy gap to generate an electron–hole pairs [10]. Several catalysts have been reported for the photocatalyst process, including TiO2 [10], ZnO [11], SnO2 [12], WO3 [13], and CuO [14].
ZnO is a nontoxic n-type semiconductor. It is a favorable candidate for photodegradation due to its unique characteristics, such as direct and wide band gap energy (3.37 eV), high electron mobility, high mechanical energy, high electrochemical stability, high thermal energy, and better luminescence property, high stability under high energy radiation [15]. Moreover, ZnO is cheap, safe and can be easily synthesized. US FDA has enlisted ZnO as generally recognized as safe metal oxide [16]. ZnO has various biomedical applications, including anticancer [17], antidiabetic [18], antibacterial [19], antifungal [20], and anti-inflammatory properties [21].
The spectral response of ZnO to the visible-light region can be altered by the type of dopants, whether metal or nonmetal atoms. The doping can significantly alter the electronic structure of semiconductors by adding localized electronic energy level under the conduction band to trap the photogenerated charge carriers and hence the photodegradation efficiency enhanced [22].
Numerous studies have reported the doping of nonmetals into the ZnO lattice to substitute lattice oxygen atoms. For effective doping, doping elements must satisfy two conditions: they must have an atomic radius that is similar to that of oxygen and have a lower electronegativity than oxygen [23]. Sun et al. [24] investigated the activity of N doped ZnO particles in the photodegradation of methyl orange (MO) dye. They showed that the efficiency of doped samples is greater than that of pure ZnO. Rajbongshi et al. [25] proposed an increase in the photocatalytic efficiency of N doped ZnO by formation of defect states in the structure of the doped samples. Zhang et al. [26] investigated the impact of sulfur doping in improving the photodegradation efficiency. They revealed that the improvement in the photocatalytic percent is correlated to introducing of oxygen vacancies and Zn interstitials into the ZnO crystal lattice besides decreasing of e−/h+ recombination.
Conventional synthesis techniques used mainly toxic chemicals, and sophisticated equipment. Thus, green synthesis has been developed, which mostly uses plants, algae, or microorganisms in place of other agents, to overcome the drawbacks of conventional techniques [27, 28]. Some studies have used green extracts, such as Capparis zeylanica [29], Viscum album [30], Prosopis juliflora [31], Nephelium lappaceum L. [32], and Clitoria ternatea Linn [33], to synthesize ZnO with photocatalytic applications. Algae are the only living organisms called nanofactories because both living and dead dried biomass have been used to synthesize various types of NPs including ZnO [34]. However, the synthesis of metal oxide and semiconductor NPs using algae has only been reported in a few numbers of literature. Consequently, we investigated the photocatalytic efficiency of biosynthesized ZnO and C doped ZnO using brown alga Cystoseira crinite.
2 Experimental Methodology
2.1 Materials
Zinc acetate dihydrate Zn(CH3COO)2⋅2H2O, glucose (C6H12O6), ethanol, brown alga Cystoseira crinite, sodium hydroxide (NaOH), distilled water, crystal violet (CV) dye (C25H30ClN3), and MO dye (C14H14N3NaO3S).
2.2 Preparation of the Extract
The collected algal samples were washed with tap, and detergent water, and finally rinsed with double distilled water to remove any foreign material; then, the freshly cleaned brown alga was left to dry in a closed room (25–28 °C) for about 30 days. The powdered samples can be obtained by grinding the dried brown alga, which is then stored in an air-tight container.
The aqueous extract was obtained by adding 1000 ml double distilled water to 15 g powdered brown alga in a beaker for 24 h, and consequently, the clear double distilled water turns dark yellow. The mixture was filtered using Whatman filter paper no.1 and stored in a freezer for further use [35, 36].
2.3 Biosynthesis of ZnO
The ZnO catalyst was synthesized using the biosynthesis method [36,37,38,39]. First, zinc acetate dihydrate salt was mixed with the prepared extract and stirred for 15 min. Then, an aqueous sodium hydroxide solution was added dropwise to the metal salt solution under continuous stirring until the pH was adjusted to 8–9. The obtained pale brown gel solution was magnetically stirred for 2 h, allowed to settle overnight at room temperature, and the supernatant liquid was discarded. Subsequently, the gel was washed severally with distilled water and ethanol, and further dried at 80 °C overnight in an oven. Finally, the desired green-synthesized ZnO catalyst was synthesized by calcining the dried, ground powder at 400 °C, 500 °C, and 600 °C for 2 h with a heating rate of 10 °C/min.
2.3.1 Biosynthesis of C/ZnO
For the green-synthesized 1%, 3%, 5%, 7%, and 9% C-doped ZnO, an appropriate amount of glucose was dissolved in the synthesized extract, added dropwise to the synthesized gel solution, and stirred vigorously and continuously for 2 h. The gel solution was then left to settle overnight at room temperature, the supernatant liquid was discarded, and the gel was washed severally with distilled water and ethanol. The gel was dried at 80 °C overnight in an oven and finally calcined at 400 °C, 500 °C, and 600 °C for 2 h at a heating rate of 10 °C/min to fabricate C-doped ZnO samples.
3 Characterization
3.1 XRD Analysis
X-ray diffraction (XRD) was used to determine the phase compositions of the samples [40]. The powder diffraction patterns were registered on the XRD PW 150 (Philips) using Ni filtered Cu Kα radiation (λ = 1.540 Å) at 40 kV, 30 mA, and a 2θ scanning range of 18–80°.
The crystallite size (nm) was determined by the Scherrer equation [41].
3.2 Microscopic Techniques
The image of the catalyst surface was investigated using the Jeol-JSM-6510LV scanning electron microscope (SEM). Before SEM analysis, an extremely small amount of the catalyst was placed on a gold-coated grid [42,43,44].
Energy-dispersive X-ray (EDX) analysis is an analytical method to characterize the elemental composition and chemical characteristics of the samples. EDX analysis depends on an energy-dispersive X-ray spectrometer connected to SEM [45, 46].
3.3 Fourier Transform-Infrared Spectra (FTIR)
FTIR technique is utilized to investigate organic, inorganic, and polymeric substances by scanning the samples with infrared light [47, 48]. The FTIR spectra of the prepared samples were examined by a Nicolet Magna-IR 550 spectrometer with a 4 cm−1 resolution and 128 scans in the mid-IR region (400–4000 cm−1). The catalyst was ground with KBr, pressed into a thin wafer, and placed inside the IR cell before recording the spectrum [49].
3.4 UV–Visible Absorption Spectroscopy
UV–vis absorption spectroscopy is a significant tool for investigating the optical characteristics of the nanoparticles. The absorbance relies on different factors like the band gap, oxygen deficiency, size and structure of NPs [50]. The band gap of the synthesized samples was obtained from the values of absorbance and wavelength using UV–vis diffuse reflectance spectroscopy (ATI Unicam UV/Visible Vision Software V3.20). The optical band gap is recorded using the Tauc equation [51]. Eg is evaluated from the intercept of the linear portion of the curve in the plot of (αhʋ)1/n on the Y-axis versus photon energy (hʋ) on the X-axis [52].
4 Estimation the Photodegradation Efficiency
The photocatalytic activity of the samples was investigated by the photodegradation of CV and MO dyes as a model of cationic and anionic dyes, respectively under UV/vis irradiation where a 400-W Hg lamp was placed 20 cm from the reaction mixture and the temperature was kept below 35 °C. Table 1 summarizes the characteristics of CV and MO dyes. The photocatalyst was suspended in a 50 ml aqueous solution of each dye (Co = 10 mgL−1). The suspension was magnetically stirred for 60 min in a dark place to attain absorption–desorption equilibrium before turning the light on. Then, the photocatalyst was separated by centrifuging 1 ml of the mixture at 4000 rpm at given intervals of illumination. Thereafter, the supernatant was investigated using a UV–vis spectrometer at λmax = 590 and 464 nm to calculate the absorbance of CV and MO solutions, respectively.
The degradation rate (%) was evaluated using the following equation [53]:
where D is the rate of degradation, (%) and Co and Ct are the dye concentration at t = 0 and t = t, respectively.
5 Results
5.1 XRD
Figures 1 and 2 reveals the XRD patterns of biosynthesized ZnO and C-doped ZnO calcined at 500 °C. The samples show the characteristic diffraction peaks located at 2θ values of 31.70°, 34.40°, 36.30°, 47.50°, 56.60°, 62.90°, 67.90°, and 69.10° and they are respectively indexed to the (100), (002), (101), (102), (110), (103), (112), and (201) lattice of hexagonal wurtzite ZnO structure (JCPDS No. 36-1451) [31, 58]. These peaks were correlated with the peaks of C-doped ZnO samples, demonstrating that the biosynthesized C-doped ZnO samples have a hexagonal wurtzite structure. The obtained results showed a variation in the crystal size due to the effect of carbon doping. The crystallite sizes of pure ZnO, 3%C/ZnO, 5%C/ZnO, and 9%C/ZnO calculated from the obtained XRD patterns were 40.3, 53.1, 44.1, and 60.6 nm, respectively. The results indicated an increase in the crystallite size due to the expansion of the lattice structure.
Figure 3 reveals the XRD patterns of biosynthesized 5%C/ZnO calcined at 400 °C, 500 °C, and 600 °C. The crystallite sizes of 5%C/ZnO calcined at 400 °C, 500 °C, and 600 °C were 28.4, 44.1, and 45.1 nm, respectively. The obtained results exhibit that the crystal size increases as the calcination temperature increases.
We can conclude that the XRD results confirmed the hexagonal structure of the biosynthesized ZnO and C/ZnO photocatalysts. They reveal sharp and intense peaks, demonstrating the good crystallinity of the catalysts. The observed crystallite size of the investigated samples indicated an increase due to the effect of carbon doping. Moreover, increasing temperature increases the crystal size.
5.2 SEM
Figure 4 shows the images used to predict the morphology of the pure ZnO and C-doped ZnO of varied concentrations. The particles of the biosynthesized samples are spherical with agglomeration. They are generally random and not uniform. These agglomerations may be due to the high surface energy of ZnO NPs and the narrow space between particles that results from the synthesis of NPs in aqueous extracts [59, 60].
Chan et al. [33] reported agglomerated ZnO NPs. They attributed the morphology of the synthesized samples to the high surface energy of ZnO during the biosynthesis in an aqueous extract of Clitoria ternatea. Linn. S. Azizi et al. [61] synthesized ZnO NPs using brown macroalgae Sargassum muticum aqueous extract. They reported that the formed ZnO was agglomerated with a hexagonal structure and that this agglomeration is caused by the polarity and electrostatic attraction of ZnO NPs.
5.3 EDX
Figure 5 reveals the EDX analysis of the biosynthesized C doped ZnO. This analysis shows the carbon as a new element into ZnO, besides there are no undesired elements in the formation of C doped ZnO.
5.4 FTIR
FTIR spectrometry was performed on the functional groups associated with ZnO and C-doped ZnO formation. Figure 6 exhibits the FTIR spectra of the biosynthesized ZnO and C-doped ZnO calcined at 500 °C. The bond observed at 3422 cm−1 is attributed to hydroxyl groups. The peak at 1802 cm−1 related to the C=O in the biosynthesized C-doped ZnO [62, 63]. The band at 1564 cm−1 is attributed to the stretching vibration of the (NH) C=O group characteristic of proteins. The peak at 1378 cm−1 suggests the CH-aliphatic group. The peak observed at 1046 cm−1 related to the C–N stretching vibrations of aromatic and aliphatic amines. The peaks lower than 1000 cm−1 are characteristic of absorption peaks of the Zn–O bond. The sharp peak below 600 cm−1 is related to the stretching frequency of the Zn–O bond in zinc oxide. This proves that the samples calcined at 500 °C produces ZnO. The FTIR spectra confirms that phenolic compounds and protein molecules are presented in the Cystoseira crinite extract, and they have a role in capping and stabilization of the biosynthesized C doped ZnO as reported in references [34, 61, 64].
5.5 UV–Visible Absorption Spectroscopy
The optical properties of the biosynthesized samples were examined by UV–vis spectroscopy. Figure 7a exhibits the UV–vis absorption spectra of the biosynthesized ZnO and 5%C-doped ZnO calcined at 500 °C. The absorption spectrum of C-doped ZnO slightly shifted to a higher wavelength than that of pure ZnO. Doping with carbon may introduce a new energy level in the band gap, which shifts the absorption band to a higher wavelength region by narrowing its band gap [65]. The optical band gap of the photocatalysts can be calculated from the plot of (αhυ)2 versus the photon energy (hυ) [66], as shown in Fig. 7b. The band gaps of ZnO and 5%C-doped ZnO calcined at 500 °C are 3.09 and 2.93, respectively. A reduction in the band gap is observed because the dopant creates a defect site and produces an electron–hole pair when exposed to light. This new energy level makes the energy needed to move from the VB to the CB lower than it would be for pure ZnO [33, 67].
6 Photocatalytic Activity
The photodegradation efficiency of ZnO and C-doped ZnO photocatalysts was investigated by the photodegradation of CV and MO dyes as a model of cationic and anionic dyes, respectively.
6.1 Effect of Carbon Concentration
Figure 8 shows the photocatalytic activity of ZnO and C-doped ZnO with varied carbon concentrations. The photodegradation of CV and MO dyes increased by increasing the carbon content up to 5%, then subsequently decreased. The photocatalytic efficiency for CV and MO dyes reached 92.7% and 87.8%, respectively, at 5%C-doped ZnO, which is higher than that of the other synthesized C-doped ZnO samples. These results were correlated with the XRD, SEM, and band gap results. The improved photocatalytic activity is ascribed to the carbon dopant which creates a defect site and produces an electron–hole pair when exposed to light. This new energy level makes the energy needed to move from the VB to the CB lower than it would be for pure ZnO [68].
6.2 Effect of Calcination Temperature
Figure 9 shows the photodegradation efficiency of 5%C-doped ZnO at different calcination temperatures (400 °C, 500 °C, and 600 °C). The photodegradation rate of CV and MO dyes over 5%C-doped ZnO initially increased, then decreased as the calcination temperature increased. The photodegradation rate showed optimum calcination temperature at 500 °C. The photodegradation efficiency of the 5%C-doped ZnO photocatalyst for both CV and MO dyes changed in the following order: 500 °C > 400 °C > 600 °C. This may be related to the photocatalyst's crystallinity, particle size, and band gap. Ansari et al. [69] also reported that C-doped ZnO calcined at 500 °C showed optimum photocatalytic performance.
6.3 Effect of the Initial pH Solution
The dye's pH is a critical operating parameter that affects photodegradation processes. Figure 10 shows the photodegradation rate of CV and MO dyes at pH levels of 2.0, 4.0, 6.0, 8.0, and 10.0, which were adjusted using HCl and NaOH. The photodegradation rate of CV dye improves at high pH values and decreases at low pH values. The photocatalyst surface becomes negatively charged at high pH values, improving the electrostatic attraction between the cationic CV dye and the photocatalyst's negatively charged surface; thus, increasing the adsorption ability and the photodegradation activity. Alternatively, at low pH values, there is a repulsive force between the cationic CV dye and the photocatalyst's positively charged surface; thus, decreasing the adsorption ability and photodegradation process [70, 71]. Similar explanation was reported by Moeen et al. [72].
In the case of MO dye, high photocatalytic activity was observed at low pH values because of the electrostatic attraction between the dye anions and the positively charged photocatalyst surface, increasing the adsorption and photodegradation. Alternatively, at the high pH values, electrostatic repulsion takes place between anions of the dye and the negatively charged photocatalyst surface, causing negligible adsorption and photodegradation. The adsorption of anionic dye on the surface of the photocatalyst is improved if it contains sulfonic groups which readily ionized in an acidic solution to produce anionic dyes. Similar results were recorded by Thomas et al. [73], Arshadi et al. [57], and Abbasi et al. [74].
6.4 Effect of Photocatalyst Content
Figure 11 exhibits the photocatalytic activity of CV and MO dyes in the presence of different photocatalyst contents (25–75 mg). The photodegradation efficiency of CV and MO dyes increases from 84.45% and 78.37% to 100% and 100%, respectively, by increasing the photocatalyst content from 25 to 75 mg. This is attributed to a rise in the active sites number as the photocatalyst content increases; thus, increasing the adsorption of dye molecules and photons from light irradiation, which increases the formation of radicals. Therefore, a high photocatalyst content can increase the photodegradation efficiency of CV and MO dyes. Similar results have been recorded in other studies [75, 76].
6.5 Effect of the Initial Dye Concentration
Figure 12 exhibits the photocatalytic activity of varied initial dye concentrations. The initial concentrations of CV and MO dyes were altered from 10 to 50 mg/L to investigate the effect of initial dye concentrations on the photocatalytic performance. The results showed that the photodegradation of CV and MO dyes decreased from 100% and 87.83% to 68.84% and 29.67%, respectively, as the initial concentration was increased up to 50 mg/L. This could be explained by an increase in dye molecules adhering to the photocatalyst surface due to an increase in the initial dye concentration. The adsorption of O2 and OH− on the photocatalyst decreases when large number of active sites are occupied by the dye molecules; thus, reducing the radicals' generation. Further, the photons were blocked before reaching the photocatalyst surface and then the photons' adsorption was reduced. Consequently, the photocatalytic activity decreases at high initial dye concentrations [57, 77,78,79].
6.6 Photocatalytic Kinetic Study
The kinetic study of the photodegradation of CV and MO dyes was performed using Langmuir–Hinshelwood kinetic model using the following equation [80, 81]:
where Co is the initial dye concentration (mg/L), C is the dye concentration (mg/L) at different irradiation times (t), and k is the photodegradation rate constant (min−1). Langmuir–Hinshelwood rate expression was utilized to investigate the relationship between the initial degradation rate and the organic substrate's initial concentration in the heterogeneous photocatalytic degradation. Figure 13 shows the plot of ln (Co/C) versus time. The observed linear fit between ln (Co/Ct) and the reaction time of varied dyes follows the first-order kinetics [82, 83]. Table 2 summarizes the photodegradation rate constant (k), half-life time (t1/2), and correlation coefficients (R2). The photocatalytic reaction rate constants follow the sequence of the photocatalytic activity. All correlation coefficients (R2) were close to 1, indicating that the dyes photodegradation fits accurately with the kinetic model. The value of t1/2 is inversely proportional to the rate constant, and hence t1/2 decreased as the rate constant increased. The photodegradation percent improved as the carbon concentration was increased up to 5% [80].
7 Mechanism of the Photodegradation Process
According to the above reported results, an expected mechanism is suggested to explain the improvement of the photocatalytic activity of C doped ZnO, as shown in Fig. 14. Improvement in the photodegradation efficiency of C doped ZnO is attributed to the separation efficiencies of photoinduced electron–hole pairs resulting from the excitation of the semiconductor. The C doping in the ZnO lattice results in the formation of new mid gap energy states above the VB of ZnO and reduces the VB value. Consequently, ZnO's band gap decreases and its light absorption increases, which favors the generated electrons’ easy migration to the CB [84].
C-doped ZnO can be excited when exposed to light, promoting the migration of VB electrons to the CB while leaving holes (h+) in the VB. The photogenerated electrons would react with the adsorbed O2 to form O2.−. However, the holes left in the VB of the C-doped ZnO would react with adsorbed OH− or water molecules to form.OH. The generated O2.−,.OH, and h+ can mineralize the dye molecules [85]. The photocatalytic mechanism can be explained using the following reaction steps [86, 87]:
Primary radicals formed by VB holes:
O2 acts as an electron scavenger in the system:
Formation of diverse peroxide species:
8 Conclusion
ZnO and C-doped ZnO with different concentrations of carbon were biosynthesized using an aqueous solution of brown alga Cystoseira crinite. XRD analysis confirmed the hexagonal structure of the biosynthesized samples. SEM images exhibited that the biosynthesized samples are spherical with agglomeration. The EDX technique revealed that carbon was successfully introduced into the ZnO structure. FTIR spectra showed phenolic compounds and protein molecules in the Cystoseira crinite extract, and these biomolecules play a role in capping and stabilizing the new formed nanoparticles. All characterization results confirmed the formation of ZnO and their properties. The carbon decreased the band gap of ZnO and played an important role in improving the photocatalytic efficiency. The photocatalytic efficiency was investigated by the photodegradation of CV and MO dyes. 5%C-doped ZnO calcined at 500 °C showed an optimum photocatalytic efficiency of 92.7% and 87.8% for CV and MO dyes, respectively. The high photocatalytic activity of CV dye was observed at a high pH value, whereas a low pH value was preferred for the high photodegradation efficiency of MO dye. The photodegradation efficiency increased as the photocatalyst content increased from 25 to 75 mg. The results show that the photocatalytic activity decreased at high initial dye concentrations. The photodegradation of CV and MO dyes decreased from 100% and 87.83% to 68.84% and 29.67%, respectively, when the initial concentration of the dye increased up to 50 mg/L. The kinetic study showed that the photodegradation of CV and MO dyes fit accurately with the first-order kinetics with correlation coefficient values close to 1. Alternatively, the value of t1/2 is inversely proportional to the rate constant, and the photodegradation rate was improved by increasing the carbon concentration up to 5%. This work provides a simple, and biosynthesis method using the brown alga Cystoseira crinite extract which is limited in researches as an alternative for the preparation of C doped ZnO with an efficient photocatalytic activity.
References
Y. Yulizar, I. Abdullah, R.M. Surya, N.L. Alifa, Green synthesis of novel YMnO3-doped TiO2 for enhanced visible-light- driven photocatalytic degradation of malachite green. J. Environ. Manag. 342, 118139 (2023)
M. Ikram, A. Haider, M. Imran, J. Haider, S. Naz, A. Ul-Hamid, A. Shahzadi, K. Ghazanfar, W. Nabgan, S. Moeen, S. Ali, Assessment of catalytic, antimicrobial and molecular docking analysis of starch-grafted polyacrylic acid doped BaO nanostructures. Int. J. Biol. Macromol. 230, 123190 (2023)
Y. Peng, Y. Zhang, H. Huang, C. Zhong, Flexibility induced high-performance MOF-based adsorbent for nitroimidazole antibiotics capture. Chem. Eng. J. 333, 678–685 (2018)
M. Ikram, A. Haider, M. Imran, J. Haider, S. Naz, A. Ul-Hamid, A. Shahzadi, S. Moeen, G. Nazir, W. Nabgan, A. Bashir, S. Ali, Cellulose grafted poly acrylic acid doped manganese oxide nanorods as novel platform for catalytic, antibacterial activity and molecular docking analysis. Surf. Interfaces 37, 102710 (2023)
N. Manavi, A.S. Kazemi, B. Bonakdarpour, The development of aerobic granules from conventional activated sludge under anaerobic-aerobic cycles and their adaptation for treatment of dyeing wastewater. Chem. Eng. J. 312, 375–384 (2017)
Z. Cai, Y. Sun, W. Liu, F. Pan, P. Sun, J. Fu, An overview of nanomaterials applied for removing dyes from wastewater. Environ. Sci. Pollut. Res. Int. 24(19), 15882–15904 (2017)
M. Ayesha-Imran, A. Haider, I. Shahzadi, S. Moeen, A. Ul-Hamid, W. Nabgan, A. Shahzadi, T. Alshahrani, M. Ikram, Polyvinylpyrrolidone and chitosan-coated magnetite (Fe3O4) nanoparticles for catalytic and antimicrobial activity with molecular docking analysis. J. Environ. Chem. Eng. 11(3), 110088 (2023)
S. Mandal, S. Natarajan, Adsorption and catalytic degradation of organic dyes in water using ZnO/ZnxFe3–xO4 mixed oxides. J. Environ. Chem. Eng. 3(2), 1185–1193 (2015)
F. Jamal, A. Rafique, S. Moeen, J. Haider, W. Nabgan, A. Haider, M. Imran, G. Nazir, M. Alhassan, M. Ikram, Q. Khan, G. Ali, M. Khan, W. Ahmad, M. Maqbool, Review of metal sulfide nanostructures and their applications. ACS Appl. Nano Mater. 6(9), 7077–7106 (2023)
E. Nyankson, R. Amedalor, G. Chandrabose, M. Coto, S. Krishnamurthy, R.V. Kumar, Microwave- and formaldehyde-assisted synthesis of Ag-Ag3PO4 with enhanced photocatalytic activity for the degradation of rhodamine B dye and crude oil fractions. ACS Omega 5(23), 13641–13655 (2020)
S.A.E. Hakam, S.M. El-Dafrawy, S. Fawzy, S.M. Hassan, Structural, photocatalytic and antibacterial activity of ZnO and ZrO2 doped ZnO nanoparticles. Int. J. Sci. Res. 3, 779–789 (2014)
A. Bhattacharjee, M. Ahmaruzzaman, A green approach for the synthesis of SnO2 nanoparticles and its application in the reduction of p-nitrophenol. Mater. Lett. 157, 260–264 (2015)
M.S. Amer, P. Arunachalam, A.M. Al-Mayouf, S. Prasad, M.N. Alshalwi, M.A. Ghanem, Mesoporous tungsten trioxide photoanodes modified with nitrogen-doped carbon quantum dots for enhanced oxygen evolution photo-reaction. Nanomaterials (Basel) 9(10), 1–19 (2019)
C. Karthikeyan, S. Karuppuchamy, Synthesis of novel CuO–Al2O3 catalyst for biodiesel production. Adv. Sci. Eng. Med. 9(12), 1011–1016 (2017)
R.M. Surya, S. Mauliddiyah, D.O. Apriandanu, Y. Yulizar, SmMnO3-decorated ZnO in a hexane-water interface for enhancing visible light-driven photocatalytic degradation of malachite green. Chemosphere 304, 135125 (2022)
J. Pulit-Prociak, J. Chwastowski, A. Kucharski, M. Banach, Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 385, 543–553 (2016)
P.C. Nagajyothi, P. Muthuraman, C.O. Tettey, K. Yoo, J. Shim, In vitro anticancer activity of eco-friendly synthesized ZnO/Ag nanocomposites. Ceram. Int. 47(24), 34940–34948 (2021)
K. Senthilkannan, A. Jenecius Alphonse, K. Sankar, K. Venkatachalam, S. Gayathri Devi, Anti-diabetic activites of ZnO doped with Ce (5 at %) nano particles (NPs). Mater. Today: Proc. 33, 3056–3057 (2020)
A. Yavaş, S. Güler, G. Onak, M. Erol, M. Torman Kayalar, O. Karaman, I. Doğan Tunç, S. Oğuzlar, Li-doped ZnO nanowires on flexible carbon fibers as highly efficient hybrid antibacterial structures. J. Alloys Compds. 891, 1–10 (2022)
A.P. Ayanwale, B.L. Estrada-Capetillo, S.Y. Reyes-López, Antifungal activity and cytotoxicity study of ZrO2-ZnO bimetallic nanoparticles. Inorg. Chem. Commun. 134, 1–9 (2021)
B.S. Surendra, C. Mallikarjunaswamy, S. Pramila, N.D. Rekha, Bio-mediated synthesis of ZnO nanoparticles using Lantana Camara flower extract: Its characterizations, photocatalytic, electrochemical and anti-inflammatory applications. Environ. Nanotechnol. Monit. Manag. 15, 1–10 (2021)
K. Qi, B. Cheng, J. Yu, W. Ho, Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compds. 727, 792–820 (2017)
S.R. Kadam, V.R. Mate, R.P. Panmand, L.K. Nikam, M.V. Kulkarni, R.S. Sonawane, B.B. Kale, A green process for efficient lignin (biomass) degradation and hydrogen production via water splitting using nanostructured C, N, S-doped ZnO under solar light. RSC Adv. 4(105), 60626–60635 (2014)
S. Sun, X. Chang, X. Li, Z. Li, Synthesis of N-doped ZnO nanoparticles with improved photocatalytical activity. Ceram. Int. 39(5), 5197–5203 (2013)
B.M. Rajbongshi, A. Ramchiary, S.K. Samdarshi, Influence of N-doping on photocatalytic activity of ZnO nanoparticles under visible light irradiation. Mater. Lett. 134, 111–114 (2014)
H. Zhang, Z. Tao, W. Xu, S. Lu, F. Yuan, First-principles study of dopants and defects in S-doped ZnO and its effect on photocatalytic activity. Comput. Mater. Sci. 58, 119–124 (2012)
C.A. Soto-Robles, O. Nava, L. Cornejo, E. Lugo-Medina, A.R. Vilchis-Nestor, A. Castro-Beltrán, P.A. Luque, Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct. 1225, 1–7 (2021)
Y. Yulizar, D.O. Apriandanu, R.M. Surya, Fabrication of novel SnWO4/ZnO using Muntingia calabura L. leaf extract with enhanced photocatalytic methylene blue degradation under visible light irradiation. Ceram. Int. 48(3), 3564–3577 (2022)
M. Nilavukkarasi, S. Vijayakumar, S. Prathipkumar, Capparis zeylanica mediated bio-synthesized ZnO nanoparticles as antimicrobial, photocatalytic and anti-cancer applications. Mater. Sci. Energy Technol. 3, 335–343 (2020)
M.H. Sayadi, S. Ghollasimood, N. Ahmadpour, S. Homaeigohar, Biosynthesis of the ZnO/SnO2 nanoparticles and characterization of their photocatalytic potential for removal of organic water pollutants. J. Photochem. Photobiol. A 425, 1–16 (2022)
S. Sheik Mydeen, R. Raj Kumar, M. Kottaisamy, V.S. Vasantha, Biosynthesis of ZnO nanoparticles through extract from Prosopis juliflora plant leaf: Antibacterial activities and a new approach by rust-induced photocatalysis. J. Saudi Chem. Soc. 24(5), 393–406 (2020)
T. Karnan, S.A.S. Selvakumar, Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 1125, 358–365 (2016)
Y.Y. Chan, Y.L. Pang, S. Lim, C.W. Lai, A.Z. Abdullah, W.C. Chong, Biosynthesized Fe- and Ag-doped ZnO nanoparticles using aqueous extract of Clitoria ternatea Linn for enhancement of sonocatalytic degradation of Congo red. Environ. Sci. Pollut. Res. Int. 27(28), 34675–34691 (2020)
A.A.K. Elrefaey, A.D. El-Gamal, S.M. Hamed, E.F. El-belely, Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it’s antimicrobial and antioxidant activities. Egypt. J. Chem. 65(4), 231–240 (2022)
R. Alghazeer, F. Whida, E. Abduelrhman, F. Gammoudi, S. Azwai, Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Nat. Sci. 05(01), 7–14 (2013)
R.A. Hamouda, W.E. Yousuf, A.B.A. Mohammed, R.S. Mohammed, D.B. Darwish, E.E. Abdeen, Comparative study between zinc oxide nanoparticles synthesis by biogenic and wet chemical methods in vivo and in vitro against Staphylococcus aureus. Microb. Pathog. 147, 1–11 (2020)
D. Antony, R. Yadav, C.S. Packiam, An Eco-approach synthesis of undoped and Mn doped ZnO nano-photocatalyst for prompt decoloration of methylene blue dye. Mater. Today: Proc. 48, 494 (2021)
J.T. Adeleke, T. Theivasanthi, M. Thiruppathi, M. Swaminathan, T. Akomolafe, A.B. Alabi, Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 455, 195–200 (2018)
O.A. Zelekew, P.A. Fufa, F.K. Sabir, A.D. Duma, Water hyacinth plant extract mediated green synthesis of Cr2O3/ZnO composite photocatalyst for the degradation of organic dye. Heliyon 7(7), 1–7 (2021)
H. Mohd Yusof, N.A. Abdul Rahman, R. Mohamad, U.H. Zaidan, A.A. Samsudin, Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 10(1), 1–13 (2020)
A.L. Patterson, The Scherrer formula for X-ray particle size determination. Phys. Rev. 56(10), 978–982 (1939)
T. Rihacek, M. Horak, T. Schachinger, F. Mika, M. Matejka, S. Kratky, T. Fort, T. Radlicka, C.W. Johnson, L. Novak, B. Sed’a, B.J. McMorran, I. Mullerova, Beam shaping and probe characterization in the scanning electron microscope. Ultramicroscopy 225, 1–9 (2021)
M. Suga, S. Asahina, Y. Sakuda, H. Kazumori, H. Nishiyama, T. Nokuo, V. Alfredsson, T. Kjellman, S.M. Stevens, H.S. Cho, M. Cho, L. Han, S. Che, M.W. Anderson, F. Schüth, H. Deng, O.M. Yaghi, Z. Liu, H.Y. Jeong, A. Stein, K. Sakamoto, R. Ryoo, O. Terasaki, Recent progress in scanning electron microscopy for the characterization of fine structural details of nano materials. Prog. Solid State Chem. 42(1–2), 1–21 (2014)
A. Bogner, P.H. Jouneau, G. Thollet, D. Basset, C. Gauthier, A history of scanning electron microscopy developments: towards “wet-STEM” imaging. Micron 38(4), 390–401 (2007)
K. Torres-Rivero, J. Bastos-Arrieta, N. Fiol, A. Florido, Chapter Ten - Metal and metal oxide nanoparticles: an integrated perspective of the green synthesis methods by natural products and waste valorization: applications and challenges. Compr. Anal. Chem. 94, 433–469 (2021)
C.O. Colpan, Y. Nalbant, M. Ercelik, 4.28 fundamentals of fuel cell technologies. Comp. Energy Syst. 4, 1107–1130 (2018)
P. Caregnato, K.R. Espinosa Jiménez, P.I. Villabrille, Ce-doped ZnO as photocatalyst for carbamazepine degradation. Catal. Today 372, 183–190 (2021)
D. Sun, K. Wang, Z. Xu, R. Li, Synthesis and photocatalytic activity of sulfate modified Nd-doped TiO2 under visible light irradiation. J. Rare Earths 33(5), 491–497 (2015)
S.M. El-Dafrawy, S.M. Hassan, M. Farag, Kinetics and mechanism of Pechmann condensation reaction over sulphated zirconia-supported zinc oxide. J. Market. Res. 9(1), 13–21 (2020)
J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang, S. Jiazhong, Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 90(12), 1773–1787 (2006)
N. Roy, S. Chakraborty, ZnO as photocatalyst: an approach to waste water treatment. Mater. Today: Proc. 46, 6399–6403 (2021)
I. Khan, S. Khan, R. Nongjai, H. Ahmed, W. Khan, Structural and optical properties of gel-combustion synthesized Zr doped ZnO nanoparticles. Opt. Mater. 35(6), 1189–1193 (2013)
M. Ikram, A. Haider, S.T. Bibi, A. Ul-Hamid, J. Haider, I. Shahzadi, W. Nabgan, S. Moeen, S. Ali, S. Goumri-Said, M.B. Kanoun, Synthesis of Al/starch co-doped in CaO nanoparticles for enhanced catalytic and antimicrobial activities: experimental and DFT approaches. RSC Adv. 12(50), 32142–32155 (2022)
J. Puneetha, N. Kottam, A. Rathna, Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg. Chem. Commun. 125, 1–12 (2021)
S. Xie, P. Huang, J.J. Kruzic, X. Zeng, H. Qian, A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 6(1), 1–10 (2016)
W. Szeto, C.W. Kan, C.W.M. Yuen, S.-W. Chan, K.H. Lam, Effective photodegradation of methyl orange using fluidized bed reactor loaded with Cross-linked chitosan embedded nano-CdS photocatalyst. Int. J. Chem. Eng. 2014, 1–16 (2014)
M. Arshadi, F. Salimi Vahid, J.W.L. Salvacion, M. Soleymanzadeh, A practical organometallic decorated nano-size SiO2–Al2O3 mixed-oxides for methyl orange removal from aqueous solution. Appl. Surf. Sci. 280, 726–736 (2013)
J. Lee, H.-S. Lim, Y.S. Kang, K.-D. Suh, Carbon-doped ZnO submicron spheres functionalized with carboxylate groups and effect of dispersion stability in the colloidal system for high photocatalytic activity. RSC Adv. 5(126), 104556–104562 (2015)
M. Amina, N.M. Al Musayeib, N.A. Alarfaj, M.F. El-Tohamy, G.A. Al-Hamoud, M.K.M. Alqenaei, The fluorescence detection of phenolic compounds in Plicosepalus curviflorus extract using biosynthesized ZnO nanoparticles and their biomedical potential. Plants (Basel) 11(3), 1–23 (2022)
J. Fowsiya, G. Madhumitha, N.A. Al-Dhabi, M.V. Arasu, Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B 162, 395–401 (2016)
S. Azizi, M.B. Ahmad, F. Namvar, R. Mohamad, Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 116, 275–277 (2014)
R.A. Rochman, S. Wahyuningsih, A.H. Ramelan, Q.A. Hanif, Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. IOP Conf. Ser. Mater. Sci. Eng. 509, 1–8 (2019)
K. Sharma, K. Maiti, N.H. Kim, D. Hui, J.H. Lee, Green synthesis of glucose-reduced graphene oxide supported Ag-Cu2O nanocomposites for the enhanced visible-light photocatalytic activity. Compos. B Eng. 138, 35–44 (2018)
A. Fouda, A.M. Eid, A. Abdelkareem, H.A. Said, E.F. El-Belely, D.H.M. Alkhalifah, K.S. Alshallash, S.E.-D. Hassan, Phyco-synthesized zinc oxide nanoparticles using marine macroalgae, ulva fasciata delile, characterization, antibacterial activity. Photocatal. Tann. Wastew. Treat. 12(7), 756 (2022)
A.B. Lavand, Y.S. Malghe, Synthesis, characterization and visible light photocatalytic activity of carbon and iron modified ZnO. J. King Saud Univ. Sci. 30(1), 65–74 (2018)
I. Halomoan, Y. Yulizar, R.M. Surya, D.O.B. Apriandanu, Facile preparation of CuO-Gd2Ti2O7 using Acmella uliginosa leaf extract for photocatalytic degradation of malachite green. Mater. Res. Bull. 150, 111726 (2022)
S.M. El-Dafrawy, M. Tarek, S. Samra, S.M. Hassan, Synthesis, photocatalytic and antidiabetic properties of ZnO/PVA nanoparticles. Sci. Rep. 11(1), 1–11 (2021)
S. Liu, C. Li, J. Yu, Q. Xiang, Improved visible-light photocatalytic activity of porous carbon self-doped ZnO nanosheet-assembled flowers. CrystEngComm 13(7), 2533–2541 (2011)
S.A. Ansari, S.G. Ansari, H. Foaud, M.H. Cho, Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 41(17), 9314–9320 (2017)
A. Kundu, A. Mondal, Photodegradation of methylene blue under direct sunbeams by synthesized anatase titania nanoparticles. SN Appl. Sci. 1(3), 1–17 (2019)
S. Alkaykh, A. Mbarek, E.E. Ali-Shattle, Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 6(4), 1–6 (2020)
S. Moeen, M. Ikram, A. Haider, J. Haider, A. Ul-Hamid, W. Nabgan, T. Shujah, M. Naz, I. Shahzadi, Comparative study of sonophotocatalytic, photocatalytic, and catalytic activities of magnesium and chitosan-doped tin oxide quantum dots. ACS Omega 7(50), 46428–46439 (2022)
M. Thomas, G.A. Naikoo, M.U.D. Sheikh, M. Bano, F. Khan, Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A 327, 33–43 (2016)
S. Abbasi, M. Hasanpour, The effect of pH on the photocatalytic degradation of methyl orange using decorated ZnO nanoparticles with SnO2 nanoparticles. J. Mater. Sci.: Mater. Electron. 28(2), 1307–1314 (2017)
Z. Zhang, Y. Xu, X. Ma, F. Li, D. Liu, Z. Chen, F. Zhang, D.D. Dionysiou, Microwave degradation of methyl orange dye in aqueous solution in the presence of nano-TiO2-supported activated carbon (supported-TiO2/AC/MW). J. Hazard. Mater. 209–210, 271–277 (2012)
S. Lan, L. Liu, R. Li, Z. Leng, S. Gan, Hierarchical hollow structure ZnO: synthesis, characterization, and highly efficient adsorption/photocatalysis toward congo red. Ind. Eng. Chem. Res. 53(8), 3131–3139 (2014)
M. Arshadi, M.K. Abdolmaleki, F. Mousavinia, A. Khalafi-Nezhad, H. Firouzabadi, A. Gil, Degradation of methyl orange by heterogeneous Fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem. Eng. Res. Des. 112, 113–121 (2016)
M. Kgatle, K. Sikhwivhilu, G. Ndlovu, N. Moloto, Degradation kinetics of methyl orange dye in water using trimetallic Fe/Cu/Ag nanoparticles. Catalysts 11(4), 1–23 (2021)
S. El-Dafrawy, S. Fawzy, S. Hassan, Preparation of modified nanoparticles of zinc oxide for removal of organic and inorganic pollutant. Trends Appl. Sci. Res. 12(1), 1–9 (2016)
S.M. El-Dafrawy, M. Farag, S.M. Hassan, Photodegradation of organic compounds using chromium oxide-doped nano-sulfated zirconia. Res. Chem. Intermed. 43(11), 6343–6365 (2017)
S. Bhatia, N. Verma, Photocatalytic activity of ZnO nanoparticles with optimization of defects. Mater. Res. Bull. 95, 468–476 (2017)
N. Verma, S. Bhatia, R.K. Bedi, Role of pH on electrical, optical and photocatalytic properties of ZnO based nanoparticles. J. Mater. Sci.: Mater. Electron. 28(13), 9788–9797 (2017)
A. Indriyani, Y. Yulizar, R.T. Yunarti, D.O. Apriandanu, R.M. Surya, One-pot green fabrication of BiFeO3 nanoparticles via Abelmoschus esculentus L. leaves extracts for photocatalytic dye degradation. Appl. Surf. Sci. 563, 150113 (2021)
A.S. Alshammari, L. Chi, X. Chen, A. Bagabas, D. Kramer, A. Alromaeh, Z. Jiang, Visible-light photocatalysis on C-doped ZnO derived from polymer-assisted pyrolysis. RSC Adv. 5(35), 27690–27698 (2015)
Q.Q. Chang, Y.W. Cui, H.H. Zhang, F. Chang, B.H. Zhu, S.Y. Yu, C-doped ZnO decorated with Au nanoparticles constructed from the metal-organic framework ZIF-8 for photodegradation of organic dyes. RSC Adv. 9(22), 12689–12695 (2019)
P.M. Perillo, M.N. Atia, C-doped ZnO nanorods for photocatalytic degradation of p-aminobenzoic acid under sunlight. Nano-Struct. & Nano-Obj. 10, 125–130 (2017)
A. Shahzadi, S. Moeen, A.D. Khan, A. Haider, J. Haider, A. Ul-Hamid, W. Nabgan, I. Shahzadi, M. Ikram, A. Al-Shanini, La-doped CeO2 quantum dots: novel dye degrader, antibacterial activity, and in silico molecular docking analysis. ACS Omega 8(9), 8605–8616 (2023)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Shady Mohamed El-Dafrawy and Mervat Farag, wrote the main manuscript text and Shady Mohamed El-Dafrawy and Mervat Farag prepared figures All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M., El-Dafrawy, S.M. & Hassan, S.M. ZnO and C/ZnO Catalysts Synthesized via Plant Mediated Extracts for Photodegradation of Crystal Violet and Methyl Orange Dyes. J Inorg Organomet Polym 34, 930–943 (2024). https://doi.org/10.1007/s10904-023-02811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02811-9