Abstract
A promising graphene polymeric composite was synthesized using gamma irradiation of reduced graphene oxide (RGO); itaconic acid (IA) grafted starch (St) and 16, 16-dimethylheptadecan-1-amine (PJMT). The prepared composite (HGPC) and RGO were characterized using different analytical methods such as FT-IR, SEM, DTA-TGA, XRD, particle size distribution, and pore size distribution. Then after, they were assessed as sorbents for removal of europium radionuclides from aqueous solutions at pH 3, volume-to-mass ratio 0.1 L g−1 and 25 °C. The sorption reaction reached the equilibrium after 4 h. The Qmax attained the values of 106.79 and 11.46 mg g−1 for Eu(III) sorption onto HGPC and RGO, respectively. The values of the isotherm parameters confirmed that a chemisorption mechanism controlled the sorption process. The calculated thermodynamic parameters demonstrated that the sorption of Eu(III) by HGPC was a spontaneous and endothermic process. 83.53% and 84.09% of europium radionuclides were effectively eluted from loaded materials using 0.1 M EDTA and 0.1 M HCl solutions, respectively.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The rapid advancement of nuclear technology and its widespread application in a variety of fields generate large quantities of hazardous radioactive species. As a result, many researchers are concerned with the treatment and disposal of active wastes. The processes the applied in removing radionuclides from aqueous solutions may include precipitation, oxidation, ion exchange, reverse osmosis, membrane electrolysis, and sorption. Even though several of these technologies provide moderate to efficient metal ion removal from aqueous solutions, sorption technology is an attractive and viable alternative. This is due to its ease of use, low cost, excellent efficiency, and extensive flexibility [1]. Radionuclide removal from aqueous systems has been extensively investigated using a variety of adsorbents such as clay minerals, metal oxides, biomaterials, and carbon nano-materials [2]. Among these sorbents, carbonic materials and their composites can be considered as potential candidates for the successful recovery of many radioisotopes [2]. As a superior adsorbent for numerous environmental applications, graphene is a two-dimensional material made primarily of sp2-hybridized carbons with only one atomic thickness [3].
Researchers have been intrigued by graphene since its discovery in 2004 because of its remarkably high surface-to-volume ratio and exceptional electrical, mechanical, thermal, and chemical properties [4,5,6]. Due to its unique and superior qualities, graphene is now used in a variety of products, such as touch screens, capacitors, spintronic devices, fuel cells, batteries, sensors, transparent conductive films, high-frequency circuits, and as a sorbent to remove radionuclides and harmful materials from aqueous solutions [7, 8].
Although graphene shows a promise in a variety of applications, two drawbacks limit its widespread use. The first is the zero-band gap, while the second is its inability to dissolve in most solvents. As a result, handling and wide usage of graphene sheets is a challenge. Due to p-p der stacking and van Waals interactions, graphene sheets tend to form irreversible agglomerates or restack to form graphite [9]. Therefore, graphene and its modified composites have found widespread application in the efficient removal of organic and inorganic contaminants from aqueous media [10,11,12]. The aforementioned problems are frequently solved by the chemically functionalizing graphene. When organic molecules on the surface of graphene are covalently attached to one another, the extended aromatic character of the material is disrupted, resulting in the formation of a band gap. Furthermore, functionalizing pristine graphene sheets with organic functional groups improves graphene dispersibilty in common organic solvents and could be a key step in the formation of graphene nano-composite materials [13]. The surface characteristics of graphene are enhanced by the imposed organic monomers. Generally, the effective separation of various metal ions from aqueous solutions was achieved using graphene hybridized with other materials. It was produced using exfoliation assisted by sonication and graphite solvothermal expansion.
Lanthanides are increasingly being used in several fields of modern industry, as well as a variety of nuclear applications [14]. As a lanthanide representative, europium is typically found in the oxidation state + 3 and, in some cases, the oxidation state + 2 [15]. Europium radioisotopes are used as burn-up monitors to assess the performance and characteristics of reactor fuels [16]. Furthermore, one of the dangerous contaminants found in radioactive wastewater is europium because of its relatively high energy (hard-emitter) and long half-life.
Numerous studies have shown that starch-grafted copolymers are effective sorbents for removal of hazardous pollutants [17,18,19,20,21]. However, no research has been conducted on starch-grafted copolymers with 1ry amine composites. Our study aims to prepare a novel composite comprises itaconic grafted starch 16, 16-dimethylheptadecan-1-amine, and graphene for 152+154Eu scavenger. The prepared composite will be characterized using different analytical techniques. Additionally, the role of parameters on 152+154 Eu(III) sorption will be explored.
2 Reagents and Methods
2.1 Reagents
None of the analytical grade compounds or reagents utilized in this investigation were further purified. Itaconic acid (IA) and graphite extra pure powders were obtained from (Merck Co., Germany). Sigma-Aldrich Co., USA provided the cross-linker methylene bis-acrylamide (MBA) and starch. BDH Chemicals Ltd. (England) manufactured potassium permanganate (KMnO4), sodium nitrate, and sulphuric acid (95–97%). Alpha Co. in India also distributed hydrogen peroxide. Bratachem Co. in Egypt supplied the ascorbic acid.
2.2 Preparation of RGO
Reduced graphene oxide (RGO) was produced by reducing graphene oxide (GO) with ascorbic acid. First, graphite was chemically oxidized to produce graphene oxide using a modified Hummers process with concentrated H2SO4 and KMnO4 [3, 22]. The prepared GO and ascorbic acid mixture was heated at 70 °C until it turned black. Decantation was used to separate the reduced graphene oxide precipitate. The precipitate was then dried for 48 h at 120 °C. The product was repeatedly washed with DDW until the water's pH reached seven [23].
2.3 Preparation of (HGPC) Composite
Itaconic acid (58%), starch (11.75%), RGO (18%), and 16, 16-dimethylheptadecan-1-amine (11.75%) monomers were cross-linked to produce the HGPC composite with a concentration of 17.5%. The monomers mixture was then exposed in a 60Co cell to gamma radiation at a dose rate of 689.538 Gy/h at the Cyclotron facility, EAEA after a 10-min ultrasonic treatment. To dissolve the unpolymerized ingredients, the freshly synthesized polymer was broken into small pieces, cleaned with acetone, and dried at 60 °C for 48 h. The dried composite was ground and stored for later experiments using an electric grinder.
2.4 Sorption Studies
The sorption of 152+154Eu radionuclides onto the prepared materials was investigated by conducting an appreciative weight of these materials in sealed glass bottles with a 5 mL solution of sorbate traced with 152+154Eu isotopes. After stirring the mixtures to the steady state, the supernatants were separated and analyzed using γ-radiometry measurements. All experiments performed three times and the represented data were the average of theses reading. As mentioned earlier, a second set of experiments was run with pH values ranging from 1.5 to 7. Furthermore, an experiment was also conducted at different initial concentrations of europium ranging from 50 to 500 mg L−1, and another was performed at different temperatures ranging from 293 to 323 K. Finally, a set of experiments were performed with of NaCl as a background electrolyte at different concentrations ranged from 0.01 to 0.2 mol L−1 to investigate the role of ionic strength. The sorbed amount (qt) (mg g−1) was calculated using the following equation:
where Ao and At are the aqueous solution initial activities and activity at a time (t), respectively. Co represents the initial concentration of Eu(III) ions, V represents the volume of solution (L), and m represents the sorbent weight (g).
2.5 Characterization
The surface’s functional groups of the applied sorbents were analyzed using a FT-IR spectrophotometer from Bomen Miclson, model MB157 (Canada). To analyze the crystallinity of sample structures, an X-ray diffraction spectrophotometer from Shimadzu, model XD-Dl-Kyoto (Japan), was utilized with a diffraction angle ranged from 4° to 70°. The thermal stability of sorbents was determined using a DTA-TGA-50, Japan, at a rate of 5 degrees Celsius per minute from room temperature to 650 °C. A Japanese JEOL JSM-5400 particle morphology analyzer was used to investigate the particle morphology of the prepared samples. A MALVERN Zetasizer Nano-Zs was used to determine the particle size of the samples. The pore size distribution and corresponding porosity were determined using a chromatech 9320 pore-sizer from the United States. The 152+154Eu activity was γ- radiometrically assayed using a well-type NaI (Tl) detector (Spectech ST 360 to crystal, USA) connected to a single-channel analyzer.
3 Results and Discussion
3.1 Composite Synthesis
The hybrid graphene polymeric composite (HGPC) was synthesized through a polymerization process aided by free radicals and triggered by gamma radiation. Before producing HGPC with specific morphology and structure, the polymerization was modified to prevent agglomeration of the reactant compound on the RGO sheets. In this regard, the amino groups of PJMT monomer reacted with the carboxylic groups of RGO utilizing IA, St, and methylene bis-acrylamide before being exposed to γ-radiation at a dose of 25 KGy [24, 25]. The free radicals initiated a polymerization process in which the reactant monomers cross-linked and grafted to the surface of graphene to form HGPC. Scheme 1 depicts the predicted interaction mechanism of the polymerization process as well as the proposed HGPC structure.
3.2 Materials Characterization
3.2.1 FT-IR Analysis
The FTIR spectra of the samples are shown in Fig. 1. The plots give rise an overview of the distinctive absorption bands that were seen in the spectra of RGO and HGPC composite before and after the sorption process. The stretching vibration band of the OH group of the various water molecules found in the samples, or the carboxylic groups on the RGO surface, could be responsible for the bands seen at 3400 and 1637 cm−1 [26]. The asymmetric and symmetric C–H stretching vibrations of the aliphatic –CH group emanating from the surface of RGO sheets may be the cause of the peaks found in the area of 2925–2851 cm−1 [27]. The C=O stretching vibration of the amide and carboxyl groups, as well as the C=O bending vibration of the carboxyl group, are mainly accountable for the peaks at 1635 cm−1 for RGO before sorption. The peak exhibited at about 1400 cm−1 is due to the carboxylate group’s symmetric stretching vibration. The peak at 1100 cm−1 might be attributed to the stretching vibration of the epoxy –C–O group [28].
3.2.2 SEM Analysis
The scanning electron microscopic (SEM) images of RGO and HGPC composite are shown in Fig. 2. As shown, RGO appears as a single layer with a heterogeneous and porous surface and no agglomeration, whereas HGPC composite exhibits a variety of morphological features and appears as layers structure and agglomeration with high roughness. The images demonstrate that RGO was dispersed homogeneously in the polymer matrix using the ultrasound waves.
3.2.3 Thermal Analysis
The thermograms obtained from the TGA analysis of the synthesized sorbents at temperatures ranging from 25 to 700 °C are shown in Fig. 3. Figure 3a depicts the thermogram of RGO and shows that there are two weight losses on the TGA profile of RGO. The first weight loss was observed at temperatures up to 150 °C and amounted to 1.51%. This weight loss could be ascribed to the evaporation of water molecules that physically adsorbed on the surface or impeded into the structural [29]. The second loss is a significant decrease detected in the temperature range of 150–400 °C. This loss was amounted to 28% and could be attributed to the removal of residual oxygenated groups on the surface of RGO, such as hydroxyl, carboxyl, and carbonyl groups. After 400 °C, RGO particles were stable at temperatures ranging from 400 to 700 °C. Figure 3b depicts the TGA analysis of the HGPC composite. In the temperature range of 160–400 °C, a weight loss of 63% was observed. This loss could be attributed to the removal of residual oxygenated groups on the surface of HGPC, such as hydroxyl, carboxyl, and carbonyl groups, as well as the combustion of organic content. Rising temperature from 400 to 520 °C resulted in a slight weight loss, amounted to 4%, could be ascribed to the scavenging of residual oxygenated groups [29]. After 400 °C, it is clear that HGPC particles were stable at the temperature ranging from 520 to 700 °C.
3.2.4 Porosity and Particle Size Measurements
Table 1 lists the surface characteristics or the prepared sorbents, including the porosity, apparent & bulk density, average pore diameter, and total pore area. The results indicate that RGO had a highly porous structure with an average pore diameter of ~ 50 nm that pointed to mesoporous, and a total pore area of 41.82 m2 g−1. The existence of mesopores on the surface of RGO could increase the possibility of interaction between the traced analyte and different functional groups on the RGO surface and hence could enhance the sorption performance of RGO as a sorbent. On the contrary, the average pore diameter in the HGPC composite was 65 nm which accounted for macro-pores [30]. Metal ions may be able to diffuse quickly through the macro-pores. The sample volume, open pores, and closed pores are all taken into account by the bulk density. While the apparent density accounts for the volume of both open and closed pores in the sample. Comparing the bulk and apparent densities of composites made of RGO and HGPC, it was found that RGO has more open pores than HGPC.
Figure 4 illustrates the particle size distribution of the prepared sorbents. RGO had fairly uniform particle sizes ranging from 140 to 190 nm, while HGPC composite had particle size distribution ranging from 425 to 750 nm. The change in size distribution could be referred to the cross-linking interaction of RGO with the polymeric matrix during forming the HGPC composite.
3.2.5 X-ray Diffraction
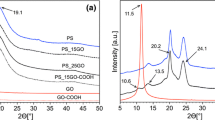
The typical XRD patterns of RGO and HGPC composite are depicted in Fig. 5. The XRD pattern of RGO had a strong and broad peak at 2θ = 26.6° corresponding to the diffraction of (002) and the characteristic diffraction peak of RGO [31]. The diffraction peaks of layered RGO had almost disappeared in HGPC. This clarified that when regular RGO stacks were exfoliated, the diffraction peaks of RGO became weakened or even disappeared [32]. Therefore, a composite composed of itaconic acid, grafted starch (St), 16, 16-dimethylheptadecan-1-amine, and RGO particles was successfully synthesized.
3.3 Sorption Studies
3.3.1 pH Impact
The variation in the aqueous solution pH value affects the durability and extent of 152+154Eu radionuclides sorption onto the RGO and HGPC materials. The variation in the amount of europium sorbed onto the surface of both RGO and HGPC samples (mg g−1) with pH value is shown in Fig. 6. The plots indicate that the retention of Eu(III) radionuclides on HGPC composite enhanced continuously with rising the initial pH value up to 3. At higher pH values, Eu(III) sorption was slightly increased until attaining a constant value at pH > 5. On the other hand, the sorption of europium on the RGO sample irregularly increased with increasing pH value to about five, after which it attained a constant value. It is important to note that the formation of Eu(OH)3 insoluble species started at pH amounted to 3.5 and sharply increased with rising pH up to 7.
The increase in europium sorption with rising pH value could be referred to the decrease in competition between [H+] and [Eu3+] to conquer the available active sites that present on the RGO, and HGPC surfaces [33]. At lower pH, the polar function group was protonated and an electrostatic repulsion between the positively charged functional groups on the surface of RGO or HGPC and Eu radionuclides interpreted the decrease in the sorption performance at lower pH [34]. Practically, the surface function groups were deprotonated by increasing pH and this resulted in a net negative charge on the surface of applied sorbents. Hence, an electrostatic attraction between Eu(III) ions and active sites on the surface of RGO or HGPC was promoted. The precipitation of Eu(III) (blank plot) as insoluble Eu(OH)3 was initiated at pH ~ 3.5, Fig. 6a. It was detected that about 11.5% of Eu(III) was precipitated at pH 4. This amount increased to ~ 90% with rising pH value of more than six. This behaviour may be due to the change in Eu(III) speciation with changing the value of pH. Further, speciation of Eu(III) at different pH values in given in Fig. 6b. The speciation of Eu(III), at different pH values, was performed using Hydra/Medusa chemical equilibrium software speciation diagram [35]. The plots clarify that the trivalent species of europium (Eu3+) prevails up to a value of pH < 6, while the ingredients EuOH2+ and Eu(OH)2+ started to appear at pH values ~ 3 and 5, respectively. At higher pH values > 6, Eu(OH)3 predominates where the removal of Eu(III) was due to precipitation only.
The change of final pH values of HGPC suspension with the initial ones is illustrated in Fig. 6c. The HGPC composite was suspended in a 0.1 mol L−1 NaNO3 at different pH values until equilibrium achieved. Then, the final pH values were determined and pHpzc values was calculated. The result exhibited a point of zero charges (pHpzc) for HGPC composite’s surface at pH 2.8. It well known that, at pH values greater than pHpzc value, the surface of the hybrid nano-composites became negative, which facilitated the adsorption of Eu(III) ions. Hence, sorption experiments were performed at pH 3 to avoid precipitation.
3.3.2 Time Impact
A series of experiment was conducted at different time intervals ranging from 5 min to 48 h to study the effect of time on the sorption of Eu(III) onto the applied sorbents and the results are displays in Fig. 7. The graphs show that the elimination of 152+154Eu radioisotopes improved as contact time rose until equilibrium was reached. Closer inspection of data illustrated that 152+154Eu radionuclides sorption occurred through three-time intervals. In the first one, 152+154Eu radionuclides were rapidly removed from the aqueous solution since the number of active locates on the applied sorbents’ surface was initially significant. The rate of 152+154Eu radionuclide sorption slowed down in the second stage due to the occupancy of active sites present on the surface of applied sorbents as well as the decrease in the concentration gradient that resulted in a decrease in the rate of 152+154Eu radionuclides transfer to the surface of used sorbents [36]. In the third stage, the removal of 152+154Eu radionuclides attained a constant value with increasing the contact time up to 48 h exhibiting a plateau. This plateau is due to reaching equilibrium and covering all active sites in the sorbent surface with 152+154Eu radionuclides. The sorbed amounts of Eu(III) onto RGO and HGPC at equilibrium were 2.91 and 4.697 mg g−1, respectively. The increase in Eu(III) entrapment by HGPC compared to RGO could be attributed to the extensive presence of a variety of functional groups on the surface of HGPC grains compared to that of RGO.
3.3.3 Concentration Impact
The sorption of 152+154Eu radionuclides onto the applied sorbents was investigated at different initial Eu(III) concentrations ranged from 50 to 500 mg L−1 and the revealed data are given in Fig. 8. The plots show that qe increased with increasing the initial concentration of 152+154Eu radionuclides. Further, HGPC exhibited a superior efficiency to retain the increased concentrations of europium compared with RGO. This high affinity could be referred to the optimum surface characteristics of HGPC that promoted the saturation extent of HGPC composed with RGO [37].
The relation between the amount adsorbed from europium onto the surface of applied sorbents and its initial concentration was mathematically modelled using the equations of four different isotherm models. These models are Langmuir [38], Freundlich [39], Dubinin–Radushkevich (D–R) [40], and the Temkin model [41]. The non-linear form of the equations of these isotherm models are:
where qmL represents the Langmuir monolayer sorption capacity (mg g−1) and KL donates the Langmuir constant related to the sorption energy. Kf represents the Freundlich sorption capacity coefficient (mg g−1), 1/n denotes the adsorption intensity constant, and qmDR represents the D–R monolayer capacity, βDR is the apparent adsorption energy constant, ε is the Polanyi potential, KT is the Temkin equilibrium constant (L g−1) corresponding to the maximum binding energy, R is a gas constant equal to 8.314 JK−1 mol−1, and T is the temperature degree (K). The sorption of Eu(III) on the applied sorbents was investigated using a key feature of the Langmuir isotherm model. This key is known as the separation factor (RL) which is a dimensionless constant denoted by the relation [42]:
The values of the RL parameter indicate the type of reaction, with the reaction being linear when RL = 1, irreversible when RL = 0, unfavourable when RL > 1, or favourable when (0 < RL < 1). Figure 9 shows a graphical representation of experimental results using the non-linear form of the equations of these isotherm models, as well as residual error. The corresponding isotherm model parameters were calculated and are scheduled in Table 2. According to the modelling data, the Freundlich model has slightly higher correlation coefficients (R2 = 0.98) for RGO than the Langmuir model (R2 = 0.921). However, for Eu(III) sorption onto the HGPC composite, the correlation coefficients (R2) of the Langmuir model were 0.985 while that of Freundlich was 0.981, respectively. As a result, the sorption mechanism of Eu(III) was regulated by Freundlich for sorption onto RGO and Langmuir for sorption onto HGPC. This result is consistent with the reduced chi-square results for RGO and HGPC, as well as the residual error plots, Fig. 9c, d. The n parameters of the Freundlich model have values ranging from 1 to 10, indicating a favourable sorption reaction [43]. Europium has a monolayer capacity of 0.349 mmol g−1 on the HGPC composite.
Isotherm non–linear fitting plots for sorption europium radionuclide on prepared RGO and HGPC sorbents: a Fitting for sorption onto RGO, b Fitting for sorption onto HGPC, c Residual error plots for isotherm modelling of sorption onto RGO, d Residual error plots for isotherm modelling of sorption onto HGPC composite
Dubinin–Radushkevich isotherm model was applied to verify the type of sorption, whether is a physical or chemical process depending on the adsorption energy value given by the equation:
If the E value is less than 8 kJ mol−1, the sorption process is controlled by physiosorption; if E is between 8 and 16 kJ mol−1, the sorption mechanism is an ion exchange process. When E is greater than 16 kJ mol−1, chemisorption may be proposed as the dominant mechanism [44]. The results in Table 2 show that chemisorption controlled the sorption of Eu(III) onto HGPC and RGO. The higher KT value of the HGPC composite than RGO was attributed to the greater binding of 152+154Eu radionuclides to the HGPC composite [45]. According to Fig. 9c, d, the residual error between predicted and experimental results demonstrates that the Freundlich model has the lowest residual error for RGO sorbent. Langmuir, on the other hand, has the lowest residual error for HGPC.
3.3.4 Ionic Strength Impact
The effect of the ionic strength of the aqueous solution on the sorption of Eu(III) was investigated using NaCl as a background electrolyte. The solution’s ionic strength was varied from 0.01 to 0.2 mol L−1, and the results are presented in Fig. 10. The results reveal that the sorption of Eu(III) onto RGO and HGPC was not affected by ionic strength. It was noted that europium uptake was slightly reduced in presence of NaCl up to 0.06 mol L−1, whereas increasing its concentration up to 0.2 mol L−1 had no influence. Generally, metal ions can act as a Lewis acid (i.e. electron acceptor) in aqueous solutions, while electron pair donating surface functional groups (such as = NH, –OH, and –COOH) can act as a Lewis base. The deprotonated functional groups (R–O−) present on the surface of the sorbent may act as a Lewis base and interact with europium ions that act as a Lewis acid (Eu(III)) to form Lewis salt-type compounds (inner-sphere complexes).
According to the previous discussion, the sorption of Eu(III) onto RGO and HGPC surfaces was dependent on europium speciation and mode of interaction. In the presence of NaCl, europium formed charged complexes (e.g. EuCl2+, EuCl2+) as well as uncharged species (e.g. EuCl3). Where they did not form ternary complexes, these chloro-species reduced the possibility of surface complexation interaction between europium and Lewis bases on sorbents surfaces. Furthermore, they have a lower affinity for applied sorbents and are less strongly sorbed than Eu(III) ions. As a result, the presence of Cl− reduced the trapping of europium ions, onto the surfaces of RGO and HGPC, from aqueous solutions containing NaCl as a background electrolyte. Additionally, Na+ ions could compete with Eu(III) ions for cation exchange sites on the sorbent surface, reducing the removal efficiency of europium ions. These findings are consistent with previous research [46]. The slight reduction in retention of Eu(III) ions in presence of NaCl electrolyte indicates a minor contribution of electrostatic sorption and confirmed the chemisorption interaction of Eu(III) with the active sites on the sorbent surface.
3.3.5 Temperature Impact
The role of temperature on the uptake of europium from an aqueous solution onto the applied sorbents (RGO and HGPC) was investigated at various temperatures ranging from 293 to 323 K, and the data are presented in Fig. 11a. The curves show that the europium sorption enhanced with increasing temperature. With increasing temperature from 293 to 323 K, the amount of Eu(III) sorbed onto RGO increased from 29.94 to 37.73 mg g−1. Furthermore, with the same temperature increase, the sorption onto HGPC increased from 46.84 to 48.12 mg g−1. This behaviour indicates that sorption was an endothermic process that increased with temperature because of the increase in the kinetic energy of the analyte, which promoted the transfer of europium molecules from the bulk solution to the surface of used sorbents [47].
To assess the thermodynamic practicability of the sorption practise and clarify its feature, the thermodynamic parameters for europium uptake on to the surface of the applied sorbents were evaluated. The thermodynamic parameters of Eu(III) retention on applied sorbents were calculated using the Vant Hoff equation [48].
where Kd is the distribution coefficient of europium ions and equals \(\frac{{q_{e} }}{Ce}\), ∆S° is the change in entropy (J mol−1 K−1), R is the constant of ideal gas that equals 8.314 J mol−1, T is the Kelvin absolute temperature (K) and ∆Ho is the apparent enthalpy change (kJ mol−1). A plot of ln Kd vs. 1/T was created and presented in Fig. 11b. The revealed straight lines have slopes equal to the overall system’s ∆H° value. At various temperatures, the magnitudes of the other thermodynamic parameters were calculated using Eqs. (9–10).
The values of the various parameters were determined and listed in Table 3. As the temperature rises, the negative values of ∆G° reflect the spontaneous nature and viability of the sorption process, whereas the decrease in its values indicates that Eu(III) sorption is more advantageous at high temperatures. The ∆H° attained a positive value, which could be considered an indication that the sorption of Eu(III) onto the sorbents is endothermic. The presence of increased randomness at the interface of both. Adsorbed europium ions gained more transitional entropy than sorbate ions lose, allowing for the presence of randomness in the system.
3.4 Maximum Sorption Capacity
The maximum sorption capacity (Qmax) values of Eu(III) sorption onto RGO and HGPC was experimentally evaluated by equilibrating 0.1 g of sorbent with 10 mL of Eu(III) radionuclides with initial concentrations of 50 mg L−1. Following equilibrium, the supernatant was separated and radiometrically analyzed, and the loaded sorbents were contacted with a fresh 152+154Eu solution and agitated until equilibrium. The procedure was reiterated until there was no more uptake detected. The following equation was used to calculate the maximum sorption capacity (Qmax):
where n is the total number of runs performed with new volumes, and qe is the amount sorbed (mg g−1). The maximum sorption capacity (Qmax) of both RGO and HGPC were determined and found to be 11.46 and 106.79 mg g−1, respectively. These values confirmed that the prepared HGPC composite had a high sorption capacity for europium ions compared with that of RGO sorbent.
3.5 Desorption and Regeneration Studies
Firstly, it is worth to clarify that the stability of the prepared composite was chemically checked using different acidic and alkaline media with molarities ranged from 0.1 to 0.5 M for each of acidic (HCl) and alkaline (NaOH) media. The results referred that the composite has high chemical stability in both acidic and alkaline media with a limited solubility not exceed 1 ± 0.5%.
The desorption behaviour of 152+154Eu radionuclides from loaded RGO and HGPC was checked using a variety of eluents including 0.1 M HCl, EDTA, MgCl2, and AlCl3 for 24 h. The desorption percentage was calculated using Eq. (12)
where Cdes denotes the concentration of desorbed 152+154Eu radionuclides, and Cads denotes the concentration of adsorbed 152+154Eu radionuclides. The obtained results are shown in Fig. 12. The findings show that a solution of 0.1 M EDTA was enough to elute europium from loaded sorbents. Further, a considerable elution from europium was obtained by applying 0.1 M HCl and AlCl3. The desorption percentages of europium eluted from RGO sorbent were 70.04, 82.96, 23.84, and 79.43% using 0.1 M HCl, 0.1 M EDTA, 0.1 M MgCl2, and 0.1 M AlCl3, respectively. The percentages of europium recovered from HGPC composite using the previously mentioned eluents were 84.09, 83.53, 6.53, and 78.48%, respectively. Contrary, the sorbed 152+154Eu was hardly eluted from RGO and HGPC composite using 0.1 M MgCl2. The variation in displayed desorption percentages of europium from both applied sorbents using different eluents could be referred to as changing the involved sorption mechanism and the physicochemical properties of the sorbent. The high desorption detected using EDTA could be ascribed to the high tendency of EDTA to form a 1:1 complex with Eu(III) according to the reaction:
This complexation interaction facilitates the elution of 152+154Eu from loaded surfaces of used sorbents [49]. Further, the great yield of 152+154Eu recovery with AlCl3 could be avouched to that Al(III) ions have a small ionic radius (0.53 Å), thus they can smoothly diffuse into the interior structure of both sorbent and elute Eu(III) (r = 1.07 Å) where it has an electronegativity (1.5) greater than that of Eu(III) that equals to 1.0. The little efficiency of MgCl2 as an eluent to desorb Eu(III) compared with AlCl3 may be referred to its large ionic radius (0.66 Ao) and small electronegativity (1.2) [50, 51].
The regeneration ability of HGPC composite was determined by performing many adsorption–desorption cycles. Adsorption–desorption cycle of HGPC composite was performed at the optimized sorption parameters with using 0.1 mol L−1 EDTA as an eluent. The aim was to investigate the regeneration efficiency of the HGPC composite and check its potential reuse as a promising sorbent for Eu(III) separation from aqueous solution. Figure 12b demonstrate that HGPC can be effectively recovered and reused up to seven times in sorption–desorption processes. The elution of loaded Eu(III) from HGPC composite’s surface was performed using 0.1 M EDTA that desorbed about 83.53% in first cycle. This efficiency reduced to 60.15% in the seventh cycle. The decrease in uptake percentage confirms that the chemisorption mechanism of Eu(III) onto HGPC surface.
3.6 Discussion
Based on the above findings, it was realized that the HGPC composite had an excellent capacity for europium removal. The good adsorption performance of HGPC could be attributed to its significant structural characteristics. The results showed that the HGPC had a typical layered nanostructure with high roughness, indicating that it had a good storage capacity. Europium cations could simply transfer into the composite interiors and become trapped. Furthermore, the composite was made up of a reduced graphene oxide hybridized with a polymer matrix, so it contained a wide range and a large number of hydrophilic groups. The HGPC surface roughness and functional groups allowed the Eu(III) ions to pass quickly through its layered structure, chemically interacted with its surface functional groups, and be retained within the composite grains. As a result, HGPC could be already considered an excellent candidate for RREs sorption. Given the abundance of hydrophilic groups, a better Eu(III) ion removal capacity of HGPC than RGO was expected. Furthermore, the faster adsorption can be attributed to the higher affinity of the Eu(III) ion for HGPC than for RGO.
The removal performance of HGPC was observed to be highly dependent on solution pH values and independent of ionic strength. Clearly, the efficiency of Eu(III) removal increased gradually as the pH of the solution increases, and slightly decreased when there is an increase in the concentration of NaCl as a background electrolyte. The existence of different interaction mechanisms during the sorption process could be attributed to the two inverse sorption trends. As a result, understanding such sorption mechanisms is critical because studying the sorption mechanism usually helps us understand the underlying interactions between adsorbents and adsorbates [52]. In general, the most common mechanisms in a sorption process are ion exchange, surface complexation, precipitation and electrostatic interaction, and physical adsorption. Metal ions may also create coordination bonds with nitrogen or oxygen atoms to generate strong bonds with electron-rich groups such as amino or hydroxyl groups [53].
The species distribution of Eu(III) under different pH values and HGPC surface properties strongly influenced the sorption trends of Eu(III) entrapment. As previously demonstrated, the most abundant surface functional groups on the surface of the HGPC composite were –NH2, –NH–, –CN–, –COOH, and –OH, while the dominant europium species in solution were Eu(III). Experimentally, the sorption of Eu(III) ions on HGPC caused a change in its functional groups, where some of these groups shifted or reduced and others disappeared after the sorption of the Eu(III) ions, see Fig. 13. These changes indicate that these function groups were involved in various interactions with Eu(III) ions during sorption process. Thus, the possible sorption interactions of Eu(III) ions with HGPC composite are shown by the following equations and a schematic diagram is illustrated in Fig. 14
-
a.
Ion exchange interactions
$${\text{HGPC}}{-}{\text{NH}}_{{2}} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{NH}}{-}{\text{Eu}}^{{{2} + }} + {\text{H}}^{ + }_{{{\text{aq}}}}$$(14)$${\text{HGPC}}{-}{\text{OH}} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{O}}{-}{\text{Eu}}^{{{2} + }} + {\text{H}}^{ + }_{{{\text{aq}}}}$$(15)$${\text{HGPC}}{-}{\text{COOH}} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{COO}}{-}{\text{Eu}}^{{{2} + }} + {\text{H}}^{ + }_{{{\text{aq}}}}$$(16) -
b.
Coordination interactions
$${\text{HGPC}}{-}{\text{NHCO}}{-} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{NH}}{-}{\text{Eu}}$$(17)$${\text{HGPC}}{-}{\text{NH}}{-} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{NH}}{-}{\text{Eu}}$$(18)$${\text{HGPC}}{-}{\text{CN}}{-} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{CN}}{-}{\text{Eu}}$$(19) -
c.
Electrostatic interactions
$${\text{HGPC}}{-}{\text{O}}^{-} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{O}}^{-} {-}{\text{Eu}}\left( {{\text{III}}} \right)$$(20)$${\text{HGPC}}{-}{\text{COO}}^{-} + {\text{Eu}}\left( {{\text{III}}} \right)_{{{\text{aq}} }} \to {\text{HGPC}}{-}{\text{COO}}^{-} {-}{\text{Eu}}^{{{3} + }}$$(21)
Additionally, the results showed that removing Eu(III) was an ionic strength-independent process, indicating that an inner-sphere complexation was the major mechanism for immobilizing Eu(III) onto HGPC. Furthermore, the sorption energy value (E) was detected to be larger than 16 kJ mol−1, showing that chemisorption governs the sorption of Eu(III) onto HGPC. The energy dispersive X-ray mapping spectra of HGPC loaded with Eu (III) are shown in Fig. 15. The spectrum exhibits Eu peaks in addition to the basic elements of HGPC composite, carbon, nitrogen, and oxygen bands. The spectra confirm the high performance of HGPC to retain Eu(III) ions successfully. The findings indicate that chemisorption and monolayer sorption were important in the removal of Eu(III) by the HGPC. The calculated thermodynamic parameters demonstrated that Eu(III) adsorption by HGPC was endothermic. Based on these findings, it can be concluded that HGPC appeared to be a promising candidate for REEs removal.
3.7 Comparison of Sorption Capacity
The sorption capacity of the HGPC composite was compared with other sorbents that previously reported in literature and the data are given in Table 4. It is clear that the prepared HGPC composite exhibits a high affinity toward Eu(III) ions with a reasonable sorption capacity compared to the other sorbents. Hence, in could be considered as a promising candidate for preconcentration and recovery of europium radionuclides from active solutions.
4 Conclusion
Reduced graphene oxide (RGO) and hybrid graphene polymeric composite (HGPC) solid phases were successfully synthesized and used as solid phases for preconcentration and recovery of europium radionuclides from aqueous solutions in this study. The sorption of europium radionuclide ions was studied in a batch technique under various experimental conditions. The results showed that the sorption of 152+154Eu radionuclides was pH dependent and was only slightly affected by changing the medium's ionic strength. The maximum sorption capacity of RGO and HGPC for europium radionuclides was 11.46 and 106.79 mg g−1, respectively. The desorption of europium radionuclides from loaded sorbents was studied using different eluents, with 0.1 M HCl, 0.1 M EDTA, and 0.1 M AlCl3 solutions yielding the best recovery. The sorption process was endothermic in nature, and it occurred spontaneously. Finally, the results demonstrated that the HGPC composite has a high potential for use as a cost-effective sorbent for the preconcentration and recovery of europium ions from aqueous solutions.
Data Availability
All the data used for this work are publicly available.
References
M. Liu, C. Chen, J. Hu, X. Wu, X. Wang, Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal. J. Phys. Chem. C 115(51), 25234–25240 (2011). https://doi.org/10.1021/jp208575m
E.A.A. El-Shazly, S.I. Moussa, G.A. Dakroury, Recovery of some rare-earth elements by sorption technique onto graphene oxide. J. Sustain. Metall. 8, 715–731 (2022). https://doi.org/10.1007/s40831-022-00520-0
R.R. Sheha, Z.A. Mekawy, H.H. Someda, M.K.K. Shehata, W.H. Mahmoud, Assessing the sorptive ability of synthesized graphene oxide-metal oxide composite to remove certain lanthanides. Clean 48(1), 2000348 (2020). https://doi.org/10.1002/clen.202000348
D. Han, L. Yan, W. Chen, W. Li, Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 83(2), 653–658 (2011). https://doi.org/10.1016/j.carbpol.2010.08.038
H. Roghani-Mamaqani, V. Haddadi-Asl, M. Mortezaei, K. Khezri, Furfuryl alcohol functionalized graphene nanosheets for synthesis of high carbon yield novolak composites. J. Appl. Polm. Sci. (2014). https://doi.org/10.1002/app.40273
W. Xue, J. Du, Q. Li, Y. Wang, Y. Lu, J. Fan, S. Yu, Y. Yang, Preparation, properties, and application of graphene-based materials in tissue engineering scaffolds. Tissue Eng. Part B 28(5), 1121–1136 (2022). https://doi.org/10.1089/ten.teb.2021.0127
S. Verma, K.-H. Kim, Graphene-based materials for the adsorptive removal of uranium in aqueous solutions. Environ. Int. 158, 106944 (2022). https://doi.org/10.1016/j.envint.2021.106944
A. Kommu, J.K. Singh, A review on graphene-based materials for removal of toxic pollutants from wastewater. Soft Mater. 18(2–3), 297–322 (2020). https://doi.org/10.1080/1539445x.2020.1739710
A.Y. Romanchuk, A.S. Slesarev, S.N. Kalmykov, Graphene oxide for effective radionuclide removal. Phys. Chem. 15, 2321–2327 (2013). https://doi.org/10.1039/C2CP44593J
J. Wang, B. Chen, Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 281, 379–388 (2015). https://doi.org/10.1016/j.cej.2015.06.102
C. Donga, S.B. Mishra, A.S. Abd-El-Aziz et al., Advances in graphene-based magnetic and graphene-based/TiO2 nanoparticles in the removal of heavy metals and organic pollutants from industrial wastewater. J. Inorg. Organomet. Polym. 31, 463–480 (2021). https://doi.org/10.1007/s10904-020-01679-3
W.M.A. El-Rouby, A.A. Farghali, M.A. Sadek et al., Fast removal of Sr(II) from water by graphene oxide and chitosan modified graphene oxide. J. Inorg. Organomet. Polym. 28, 2336–2349 (2018). https://doi.org/10.1007/s10904-018-0885-9
E.A. Sahar Sharaf El-Deen, S.I. Moussa, Z.A. Mekawy, K.K. Mohamed Shehata, S.A. Sadeek, H.H. Someda, Evaluation of CNTs/MnO2 composite for adsorption of 60Co(II), 65Zn(II) and Cd(II) ions from aqueous solutions. Radiochim. Acta 105(1), 43–55 (2017). https://doi.org/10.1515/ract-2016-2624
S.S. Metwally, H.E. Rizk, Preparation and characterization of nano-sized iron–titanium mixed oxide for removal of some lanthanides from aqueous solution. Sep. Sci. Technol. 49, 2426–2436 (2014). https://doi.org/10.1080/01496395.2014.926457
M.G. Kapnisti, F.G. Noli, E.S. Papastergiadis, E.G. Pavlidou, Exploration of the parameters affecting the europium removal from aqueous solutions by novel synthesized titanium phosphates. J. Environ. Chem. Eng. 6, 3408–3417 (2018). https://doi.org/10.1016/j.jece.2018.05.010
G.F. Fryxell, W. Chouyyok, R.D. Rutledge, Design and synthesis of chelating diamide sorbents for the separation of lanthanides. Inorg. Chem. Commun. 14(6), 971–974 (2011). https://doi.org/10.1016/j.inoche.2011.03.045
M. Zdanowicz, B. Schmidt, T. Spychaj, Starch graft copolymers as superabsorbents obtained via reactive extrusion processing. Pol. J. Chem. Technol. 12(2), 14–17 (2010). https://doi.org/10.2478/v10026-010-0012-3
K. Mostafa, H. Ameen, A. Ebessy, A. El-Sanabary, Harnessing of newly tailored poly (Acrylonitrile)-starch nanoparticle graft copolymer for copper ion removal via oximation reaction. Pigment Resin Technol. (2023). https://doi.org/10.21203/rs.3.rs-412347/v1
A.M. Farag, H.H. Sokker, E.M. Zayed, F.A. Nour Eldien, N.M. Abd Alrahman, Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 120, 2188–2199 (2018). https://doi.org/10.1016/j.ijbiomac.2018.06.171
Y. Chen, W. Zhao, H. Wang, X. Meng, L. Zhang, A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb(II), Cu(II), Cd(II) and Cr(III) ions from aqueous solutions. R. Soc. Open Sci. 5(6), 180281 (2018). https://doi.org/10.1098/rsos.180281
D. Soto, J. Urdaneta, K. Pernia, O. León, A. Muñoz-Bonilla, M. Fernández-García, Itaconic acid grafted starch hydrogels as metal remover: capacity, selectivity and adsorption kinetics. J. Polym. Environ. 24(4), 343–355 (2016). https://doi.org/10.1007/s10924-016-0780-9
N. Zaaba, Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng. 184, 469–477 (2017). https://doi.org/10.1016/j.proeng.2017.04.118
E. Andrijanto, S. Shoelarta, G. Subiyanto, S. Rifki, Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. AIP Conf. Proc. 1725(1), 20003 (2016). https://doi.org/10.1063/1.4945457
A.A. Eliwa, A.E. Mubark, G.A. Dakroury, E.A.A. El-Shazly, K.M. El-Azony, Polyacryl-dimethyl-heptadecanamine-mullite as a promising sorbent for chromium and vanadium sorption from ilmenite. J. Environ. Chem. Eng. 10(6), 108886 (2022). https://doi.org/10.1016/j.jece.2022.108886
A.H. Ali, G.A. Dakroury, M.S. Hagag et al., Sorption of some rare earth elements from acidic solution onto poly(acrylic acid–co-acrylamide/16, 16-dimethylheptadecan-1-amine) composite. J. Polym. Environ. 30, 1170–1188 (2022). https://doi.org/10.1007/s10924-021-02271-7
A.A. Ahribesh, S. Lazarevic, I.J. Castvan, B. Jokic, V. Spasojevic, T. Radetic, D. Janackovic, R. Petrovic, Influence of the synthesis parameters on the properties of the sepiolite-based magnetic adsorbents. Powder Technol. 305, 260–269 (2017). https://doi.org/10.1016/j.powtec.2016.09.086
Y. Fei, L. Yong, H. Sheng, M. Jie, Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 484, 196–204 (2016). https://doi.org/10.1016/j.jcis.2016.08.068
H. Wang, X. Yuan, Y. Wu, H. Huang, G. Zeng, Y. Liu, X. Wang, N. Lin, Y. Qi, Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 279, 432–440 (2013). https://doi.org/10.1016/j.apsusc.2013.04.133
S.N. Alam, N. Sharma, L. Kumar, Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6, 1–18 (2017). https://doi.org/10.4236/graphene.2017.61001
S. Kang, J.S. Yu, M. Kruk, M. Jaroniec, Synthesis of an ordered macroporous carbon with 62 nm spherical pores that exhibit unique gas adsorption properties. Chem. Commun. 16, 1670–1671 (2002). https://doi.org/10.1039/B204756j
G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu, J. Yao, Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. 112(22), 8192–8195 (2008). https://doi.org/10.1021/jp710931h
Y. Chen, H. Wang, J. Yu, Y. Wang, J. Zhu, Z. Hu, Mechanically strong and pH-responsive carboxymethyl chitosan/graphene oxide/polyacrylamide nanocomposite hydrogels with fast recoverability. J. Biomater. Sci. Polym. Ed. 28, 1899–1917 (2017). https://doi.org/10.1080/0920563.2017.1358548
G.A. Dakroury, E.A.A. El-Shazly, H.S. Hassan, Preparation and characterization of ZnO/Chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. J. Radioanal. Nucl. Chem. 330(1), 159–174 (2021). https://doi.org/10.1007/s10967-021-07935-1
K. Chang, X. Li, Q. Liao, B. Hu, J. Hu, G. Sheng, W. Linghu, Y. Huang, A.M. Asiri, K.A. Alamry, Molecular insights into the role of fulvic acid in cobalt sorption onto graphene oxide and reduced graphene oxide. Chem. Eng. J. 327, 320–327 (2017). https://doi.org/10.1016/J.CEJ.2017.06.100
C. Peng, Y. Ma, Y. Ding, X. He, P. Zhang, T. Lan, D. Wang, Z. Zhang, Z. Zhang, Influence of speciation of thorium on toxic effects to green algae chlorella pyrenoidosa. Int. J. Mol. Sci. 18(4), 795 (2017). https://doi.org/10.3390/ijms18040795
G.A. Dakroury, Sh.F. Abo-Zahra, H.S. Hassan, Utilization of olive pomace in nano MgO modification for sorption of Ni(II) and Cu(II) metal ions from aqueous solutions. Arab. J. Chem. 13(8), 6510–6522 (2020). https://doi.org/10.1016/j.arabjc.2020.06.008
E. Igberase, P. Osifo, A. Ofomaja, The adsorption of Pb, Zn, Cu, Ni, and Cd by modified ligand in a single component aqueous solution: equilibrium, kinetic, thermodynamic, and desorption studies. Int. J. Anal. Chem. (2017). https://doi.org/10.1155/2017/6150209
E. Abdel-Galil, A. Ibrahim, W. El-Kenany, Facile fabrication of a novel silico vanadate ion exchanger: evaluation of its sorption behavior towards europium and terbium ions. Desalin Water Treat (2021). https://doi.org/10.5004/dwt.2021.27261
E. Jasper, V.O. Ajibola, J.C. Onwuka, Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 10, 132 (2020). https://doi.org/10.1007/s13201-020-01218-y
M. Yadav, N.K. Singh, Isotherm investigation for the sorption of fluoride onto Bio-F: comparison of linear and non-linear regression method. Appl. Water Sci. 7, 4793–4800 (2017). https://doi.org/10.1007/s13201-017-0602-9
A. Benmessaoud, D. Nibou, E.H. Mekatel, S. Amokrane, A comparative study of the linear and non-linear methods for determination of the optimum equilibrium isotherm for adsorption of Pb2+ ions onto algerian treated clay. Iran. J. Chem. Chem. Eng. 39(4), 153–171 (2020). https://doi.org/10.30492/ijcce.2019.35116
M.K. Uddin, A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017). https://doi.org/10.1016/j.cej.2016.09.029
L. Anah, N. Astrini, Isotherm adsorption studies of Ni(II) ion removal from aqueous solutions by modified carboxymethyl cellulose hydrogel. IOP Conf. Ser. 160, 012017 (2018). https://doi.org/10.1088/1755-1315/160/1/012017
L.M. Cozmuta, A.M. Cozmuta, A. Peter, C. Nicula, E.B. Nsimba, H. Tutu, The influence of pH on the adsorption of lead by Na-clinoptilolite: kinetic and equilibrium studies. Water 38, 269–278 (2011). https://doi.org/10.4314/wsa.v38i2.13
E.A.A. Shazly, G.A. Dakroury, H.H. Someda, Kinetic and isotherm studies for the sorption of 134Cs and 60Co radionuclides onto supported titanium oxide. J. Radioanal. Nucl. Chem. 330(1), 127–139 (2021). https://doi.org/10.1007/s10967-021-07956-w
L.J. Criscenti, D.A. Sverjensky, A single-site model for divalent transition and heavy metal adsorption over a range of metal concentrations. J. Colloid Interface Sci. 253(2), 329–352 (2002). https://doi.org/10.1006/jcis.2002.8529
T.S. Badessa, E. Wakuma, A.M. Yimer, Bio-sorption for effective removal of chromium(VI) from wastewater using Moringa stenopetala seed powder (MSSP) and banana peel powder (BPP). BMC Chem. 14, 71 (2020). https://doi.org/10.1186/s13065-020-00724-z
S. Hino, T. Ichikawa, Y. Kojima, Thermodynamic properties of metal amides determined by ammonia pressure-composition isotherms. J. Chem. Thermodyn. 42(1), 140–143 (2010). https://doi.org/10.1016/j.jct.2009.07.024
M. Descostes, I. Pointeau, J. Radwan, J. Poonoosamy, J.-L. Lacour, D. Menut, T. Vercouter, R.V.H. Dagnelie, Adsorption and retarded diffusion of Eu III –EDTA—through hard clay rock. J. Hydrol. 544, 125–132 (2017). https://doi.org/10.1016/j.jhydrol.2016.11.014
S. Huang, P. Du, C. Min, Y. Liao, H. Sun, Y. Jiang, Poly(1-amino-5-chloroanthraquinone): highly selective and ultrasensitive fluorescent chemosensor for ferric ion. J. Fluoresc. 23(4), 621–627 (2013). https://doi.org/10.1007/s10895-013-1179-9
A. Mohammadinezhad, G.B. Marandi, M. Farsadrooh, H. Javadian, Synthesis of poly(acrylamide-co-itaconic acid)/MWCNTs superabsorbent hydrogel nanocomposite by ultrasound-assisted technique: swelling behavior and Pb (II) adsorption capacity. Ultrason. Sonochem. 49, 1–12 (2018). https://doi.org/10.1016/j.ultsonch.2017.12.028
H.N. Tran, D.T. Nguyen, G.T. Le, F. Tomul, E.C. Lima, S.H. Woo, A.K. Sarmah, H.Q. Nguyen, P.T. Nguyen, D.D. Nguyen, T.V. Nguyen, Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: a systematic in-depth review. J. Hazard. Mater. 373, 258–270 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.018
S.E. Cabaniss, Forward modeling of metal complexation by NOM: II. Prediction of binding site properties. Environ. Sci. Technol. 45(8), 3202–3209 (2011). https://doi.org/10.1021/es102408w
F. Granados-Correa, J. Vilchis-Granados, M. JiménezReyes, L.A. Quiroz-Granados, Adsorption behaviour of La(III) and Eu(III) ions from aqueous solutions by hydroxyapatite: kinetic, isotherm, and thermodynamic studies. J. Chem. 2013, 1–9 (2013). https://doi.org/10.1155/2013/751696
B. Xu, Y. Zhu, H. Liu, T. Chen, The kinetic and thermodynamic adsorption of Eu(III) on synthetic maghemite. J. Mol. Liq. 221, 171–178 (2016). https://doi.org/10.1016/j.moclliq.2016.05055
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
GA Dakroury: Conceptualization, Visualization, Writing-reviewing, and editing. ZAM: experimental work, editing, and reviewing. RRS: Data curation, writing—original draft review and editing. All authors contributed to the study conception and design, Material preparation, data collection, and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and is not under consideration for publication elsewhere.
Consent to Participate
All of the authors consented to participate in the drafting of this manuscript.
Consent for Publication
All of the authors consent to publish this manuscript.
Research Involving Human Participants and/or Animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dakroury, G.A., Mekawy, Z.A. & Sheha, R.R. Development of Hybrid Graphene Polymeric Composite and Its Potential Application in Europium Removal. J Inorg Organomet Polym 33, 3565–3582 (2023). https://doi.org/10.1007/s10904-023-02756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02756-z