Abstract
In the pharmaceutical market, the need to find effective systems for the efficient release of poorly bioavailable drugs is a forefront topic. The inorganic–organic hybrid materials have been recognized as one of the most promising systems. In this paper, we developed new Hydroxypapatite@Furosemide hybrids with improved dissolution rates in different media with respect to the drug alone. The hybrids formation was demonstrated by SEM/EDS measurements (showing homogeneous distribution of the elements) and FT-IR spectroscopy. The drug was adsorbed onto hydroxyapatite surfaces in amorphous form, as demonstrated by XRPD and its thermal stability was improved due to the absence, in the hybrids, of melting and decomposition peaks typical of the drug. The Sr substitution on Ca sites in hydroxyapatite allows increasing the surface area and pore volume, foreseeing a high capacity of drug loading. The dissolution tests of the hybrid compounds show dissolution rates much faster than the drug alone in different fluids, and also their solubility and wetting ability is improved in comparison to furosemide alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The search for effective systems increasing the bioavailability of drugs by improving the physico-chemical and/or biopharmaceutical properties is a fascinating and forefront topic, particularly appealing for poorly soluble drugs administered in oral form, the most common way for chronic therapies requiring repeated administrations. The design of suitable systems having complex architectures or composite materials is a quite new and appealing strategy applied in many different fields, from water purification to food and sensors [1,2,3]. In particular, in the pharmaceutical industry, the materials constituted by host inorganic matrices and drug guests have attracted considerable attention and represent one of the latest research strategies in the development of new drug alternatives [4,5,6,7,8].
Furosemide (Fur), 4-chloro-2-[(2-furanylmethyl)-amino]-5-sulfamoylbenzoic acid, is a high ceiling loop diuretic drug to treat edema and hypertension linked to congestive heart failure, cirrhosis of the liver and renal disease. According to the Biopharmaceutics Classification System (BCS), furosemide belongs to class IV drug, characterized by low solubility and permeability values [9]. Indeed, furosemide is almost insoluble in water (6 mg L−1), resulting in significant intra-individual variations in absorption and very poor oral bioavailability. It has a weak acidic nature, a short half-life (1–3 h) and poor bioavailability (60–65%) since this molecule is preferentially absorbed in the gastric mucosa and upper intestine, where the drug shows the lowest solubility (5–20 μg/ml). Fur exists in different polymorphic forms, showing variable dissolution rates [10, 11].
Despite some drawbacks, Fur shows great efficacy, and is highly used in therapeutics worldwide. However, high doses or prolonged use can lead to fluid and electrolyte imbalance which can provoke headaches, cramping, thirst, and weakness [12]. So, for the pharmaceutical industry, the development of new oral formulations is mandatory for the improvement of its release to minimise the dose size, so reducing side effects.
Numerous successful attempts were reported in the literature. Mesoporous materials [13, 14], surfactant-based methods [15], Na and K salts [16], cocrystals of Fur with a variety of co-formers, as well as hydrates and solvates [17,18,19,20] have been reported. Among the cited approaches to improve the dissolution rate of furosemide, the use of inorganic–organic hybrid materials has been recognized as one of the most promising systems.
Hydroxyapatite (HAP), Ca10(PO4)6(OH)2, is the main constituent of biological tissues such as bones and teeth. It has several intriguing features, such as good biocompatibility, biodegradability, osteogenisis, osteoconductivity and bioactivity, and can form direct bonds with living tissues [21]. In form of nanocrystals, HAP has unique properties, i.e. high surface to volume ratio, reactivity and biomimetic morphologies compared to the bulk counterpart due to quantum size effects and surface phenomena at the nanoscale. HAP nanocrystals can be used for example in tissue engineering, nanomedicine, industrial catalysis and in orthopaedic implant coating [22, 23]. In addition, HAP nanospheres, thanks to their low solubility in physiological conditions, can be used as carriers for controlled and local drug delivery both by surgical placement and injection [24, 25]. The drug delivery from hydroxyapatite minimizes the toxicity to other organs reducing the drug concentration in the blood, avoiding repeated dosage of drugs. HAP easily binds to both positive and negative molecules by simple adsorption and is used for delivery of molecules like antibiotics, contraceptives, acetylsalicylic acid, hormones, insulin and anticancer drugs [26,27,28]. The HAP doping/substitution with different kind of elements (Mg, Si, Sr, Eu, Zn, Ce) was proposed as a way to improve the drug loading [29,30,31].
In this work, we propose, for the first time, a drug-delivery system based on the adsorption of Furosemide onto Pure and Sr-substituted Hydroxyapatite. The hybrids’ characterization was performed by the combined use of X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), and Fourier transform infrared spectroscopy (FT-IR), particularly useful to prove the presence of the drug onto HAP surface/pores. Scanning electron microscopy (SEM) coupled with energy dispersive X-ray spectroscopy (EDS) microanalysis supported the formation of the hybrids. The measure of contact angle of the powders, the solubility and the in vitro dissolution tests, performed in different media simulating the gastro-intestinal environment, demonstrated the effectiveness of the hybrids for the improvement of drug wettability and dissolution rate.
2 Materials and Methods
2.1 Syntheses
2.1.1 Synthesis of Hydroxyapatite
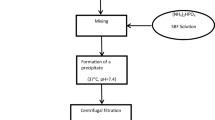
Hydroxyapatite was synthesized by means of co-precipitation method. Ca(NO3)2 4H2O and (NH4)2HPO4 in the proper stoichiometric amount were dissolved in two different beckers in distilled water (15 ml) and basified with NH4OH until pH ≈ 10 was reached. After 15 min of stirring, the solution of phosphate was poured drop by drop in that of calcium, then the pH was checked and, if necessary, adjusted to 10 with ammonium hydroxide. After 30 min of stirring, the temperature was raised to 80 °C and maintained for 1 h. After cooling to room temperature, the solution was centrifuged, and the collected powder was washed three times with deionized water, then transferred in oven at 100 °C for 22 h. This sample will be named HAP-Pure.
2.1.2 Synthesis of Sr-Doped Ca6Sr4(PO4)6 (OH)2 Hydroxyapatite
Ca(NO3)2 4H2O, Sr(NO3)2 and (NH4)2HPO4 in the proper stoichiometric amounts to obtain Ca6Sr4(PO4)6 (OH)2, were dissolved in three different beckers in distilled water (10 ml each one) and basified with NH4OH until pH ≈ 10 was reached. After 15 min of stirring, the solution of Sr was poured drop by drop in that of calcium and the pH was checked and eventually adjusted to 10 with ammonium hydroxide. Then, the solution of phosphate was poured into that of calcium/strontium with the control of pH. After 30 min of stirring, the temperature was raised to 80 °C and maintained for 1 h. At the end, after cooling to room temperature, the solution was centrifuged, and the powder washed with deionized water and treated in oven at 100 °C for 22 h. This sample will be named HAP-Sr.
2.1.3 Synthesis of HAP@Furosemide Hybrids
Furosemide (Fur), Formula C12H11ClN2O5S (Scheme 1), was gently donated by Fabbrica Italiana Sintetici S.p.A., Vicenza, Italy.
Furosemide was dissolved into a mixture of ethanol:water (3:1 ratio) (drug concentration 10 mg/ml, 10 ml overall). Then, 150 mg of HAP-Pure or HAP-Sr was added to the drug solution and maintained for 24 h at ambient temperature under continuous stirring. Then, the powder was centrifuged, dried at room temperature, and maintained in desiccator for the subsequent use. In the following, the samples will be named HAP-Pure-Fur and HAP-Sr-Fur.
2.2 Techniques
2.2.1 Physico-chemical measurements
XRPD measurements were performed by using a Bruker D5005 diffractometer (Bruker BioSpin, Fällanden, Switzerland) with the Cu Kα radiation, graphite monochromator and scintillation detector. The patterns were collected in air with a step size of 0.03° and counting time of 2 s per step in the angular range 5–70° (Hybrids), 5–35° (Fur) and 10–70° (HAPs) by using a low background silicon sample holder.
FT-IR spectra were obtained with a Nicolet FT-IR iS20 spectrometer (Nicolet, Madison, WI, USA) equipped with ATR (Attenuated Total Reflectance) sampling accessory (Smart iTR with diamond plate) by co-adding 32 scans in the 4000–650 cm−1 range at 4 cm−1 resolution.
DSC measurements were carried out by a DSC Q2000 apparatus interfaced with a TA 5000 data station (TA Instruments, New Castle, DE, USA). The instrument was calibrated using ultrapure (99.999%) indium (m.p. = 156.6 °C; ∆H = 28.54 J g−1) as standard. The calorimetric measurements were conducted up to 250 °C at a heating rate of 5 °C min−1 on samples amount of about 3–5 mg in open standard aluminium pans under nitrogen flow (45 mL·min−1).
SEM images were collected by a Zeiss Evo MA10 (Carl Zeiss, Oberkochen, Germany) microscope coupled with the EDS detector for microanalysis (X-max 50 mm, Oxford Instruments). The samples for SEM analysis were sputtered with a thin layer of gold and analysed at an acceleration voltage of the electron beam of 20 kV. The EDS data were obtained on pristine samples (not sputtered).
N2 adsorption isotherms were collected on samples previously evacuated overnight at room temperature. Use was made of a Porosimeter (Thermoelectron Model Sorptomatic 1990, Waltham, Massachusets, United States). The data were analysed with the Brunauer, Emmett, and Teller (BET) algorithm to determine the surface area and pores’ volume.
2.2.2 Pharmaceutical Measurements
The Furosemide content in the HAP-Pure-Fur and HAP-Sr-Fur samples was determined in the conditions in which the drug is highly soluble, i.e. phosphate buffer pH = 5.8. In the same conditions, a calibration curve was previously performed at 274 nm, 1 cm cell, obtaining a correlation coefficient of 0.9999. At this wavelength, the carrier Hydroxyapatite does not show any absorption.
It was not possible to determine the thermodinamic solubility of the hybrid compounds with the shake flask method, because the carrier is insoluble in water and thus it was not feasible to establish when a saturated solution of Fur was reached. So, we wheighed samples corresponding to at least four times the drug dose (100 mg L−1) in the flasks, that were maintained under magnetic stirring at 300 rpm, at 21 °C. Then we measured the Fur absorbance, after different time intervals, on filtered portion (0.45 μm) of the supernatant solution, to verify if all the quantity of the considered drug was completely dissolved.
The wettability of the samples was determined by measuring the contact angle θ (deg). Contact Angle Meter DMe-211Plus (NTG Nuova Tecnogalenica, Cernusco, Italy) was used for contact angle determination: a 9 µl drop of fluid (deionized water, pH 1.0 hydrochloric acid solution or pH 5.8 buffer) was extruded from the needle and dropped onto the solid surface of the powder. Photos were recorded at progressive times and the contact angle was measured by the software provided. Three replicas for each sample were performed. These measurements provide evidence of the attitude of the solid samples to be wetted by the considered fluids.
For the dissolution test, all the samples were sieved through a 400-mesh grid and then samples corresponding to 25 mg of Fur were weighed. The dissolution test was performed using paddle method (Erweka DT-D6, Dusseldorf, Germany) at 37.0 ± 0.5 °C, 50 rpm, in 900 mL of pH 5.8 buffer, medium required by the US Pharmacopeia [32], and in other two fluids: hydrochloric acid solution, pH 1.0, to simulate fasting gastric conditions and deionized water (pH 6.8), to simulate neutral condition (n = 3 repetitions). The dissolution media were prepared following the reagents and buffer solutions section of the USP [33].
The amount of drug dissolved was determined by UV detection, on filtered portions of the dissolution fluid, at 274 nm with a spectrophotometer (Lambda 25; Perkin-Elmer) and the data were processed by a suitable software (Winlab V6 software, Perkin-Elmer, Monza, Italy).
3 Results and Discussion
3.1 Physico-Chemical Characterization
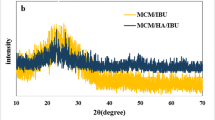
The X-ray powder diffraction patterns of pure Furosemide drug and HAPs, as well as those of HAPs@Fur hybrids are shown in Fig. 1. Furosemide is a crystalline material (Fig. 1A), whose pattern well agrees with that reported in the literature and attributed to Form I [10, 34, 35]. The HAP-Pure sample reflections (Fig. 1B) are in very good agreement with the peak positions expected for Ca10(PO4)6(OH)2 compound (Card No. 74-0565), with a hexagonal lattice and the P63/m space group. The same is true for HAP-Sr sample: its pattern is well comparable with that of HAP-Pure, apart a shift to lower angles of all the peak positions, suggesting an expansion of the lattice parameters. This is due to the difference of the ionic radii of Ca and Sr ions (1.18 and 1.31 Å with respect to 1 and 1.18 Å for the nine and six coordination environments respectively, for the two cationic sites in HAP), [36] causing variations in the crystal lattice dimensions, as commonly occurs in many systems in case of cationic substitution or formation of solid solutions [37, 38]. In addition, the pattern of HAP-Sr, with broader and less defined peaks with respect to HAP-Pure, is suggestive of a low crystallinity, notwithstanding the same synthesis conditions. An effect of the Sr ions on the kinetic of the synthesis can be supposed. The XRPD patterns of the hybrids are practically over imposable to the corresponding patterns of HAPs: all the peaks pertain to the HAP phase alone, no peaks due to Furosemide can be evidenced. This can be due to the presence of Furosemide in amorphous form. However, from these evidences, it is clear that the only XRPD data are not able to confirm the hybrids formation, but other kind of analysis, particularly spectroscopic or compositional ones, are required to prove the Furosemide presence on HAP surfaces. However, an indirect proof of the presence of Furosemide on the HAP particles could be represented by the reduction of the intensities of the reflections of the entire hybrids’ patterns with respect to the corresponding HAPs: this evidence could be related to the coverage of HAP particles with a sort of a coating layer of drug.
The spectroscopic analysis could provide useful insights into the Furosemide adsorption onto the HAP nanoparticles. The FT-IR spectra of Fur, HAP-Pure, HAP-Sr and the hybrids HAP-Pure-Fur and HAP-Sr-Fur are reported in Fig. 2.
The spectrum of the pure Furosemide drug well agrees with the literature data of the polymorphic Form I, in agreement also with XRPD findings [35]. Fur exhibits the main vibrational frequencies at 3396 and 3278 cm−1 (sulphonamide primary amine stretching), 3348 cm−1 (secondary amine stretching), 1667 cm−1 (carboxyl stretching), 1590 and 1559 cm−1 (N–H bending vibrations), and 1315 and 1139 cm−1 (SO2 asymmetric and symmetric stretching).
The HAP-Pure FT-IR spectrum too well agrees with those reported in the literature for Hydroxyapatite [39]. In this case, few bands are observed in the analysed FT-IR range: the sharp band at 3568 cm−1 is assigned to the OH stretching mode, the bands at 1088 and 1022 cm−1 are due to P-O antisymmetric stretching modes and that at 962 cm−1 to P–O symmetric stretching mode. The HAP-Sr spectrum is like that of HAP-Pure: the bands due to P–O bonds slightly shift to lower wavenumbers, due to the higher reduced mass of the harmonic oscillator related to the Sr presence. In addition, the band at 3568 cm−1 is very low, substituted by a broader band however attributable to OH vibration. This can be due to the increase of lattice parameters because of Sr substitution and the formation of an open structure about the OH groups, reducing the crystal force field and increasing the mixing of the OH vibrational and translational motions.
In the FT-IR spectra of the hybrids (Fig. 2), together with the bands previously commented of HAP-Pure and HAP-Sr samples, some small peaks attributable to the drug are visible, particularly in the range 1600–1200 cm−1. The band at about 1610 cm−1, well evident in both the samples, is compatible with the hydrogen bonds formation between HAP and Fur. Other low bands typical of the drug are also shifted with respect to those of the Fur alone, suggesting a complex hydrogen bonding scheme in the hybrid compounds.
Thermal data, in particular the melting or decomposition temperatures of drugs, can be employed to prove the formation of new compounds, because of drug adsorption [6,7,8]. The DSC curves of Fur, HAP-Pure-Fur, and HAP-Sr-Fur are reported in Fig. 3. The curve of Fur agrees with the literature, the following thermal effects are present: a small endothermic peak at about 135 °C, due to the solid–solid phase transition between Form I, the low temperature stable form, and a high temperature stable form [34], in enantiotropic relationship; a complex endothermic-exothermic thermal signal which indicates a melting-decomposition process of the drug at about 217 °C.
HAP, as other anologous inorganic compounds, has a high structural stability in this temperature range and, as expected, no thermal events are present [40].
The DSC traces of both hybrids are similar (Fig. 3): a flat curve, without any thermal event, apart a broad band at low temperature due to water desorption, is observed, proving that the Furosemide turned out to be amorphous and stable to decomposition, because of its adsorption onto the HAP surfaces.
The morphological characterization was performed by SEM microscopy. The micrographs of Fur, HAP-Pure and HAP-Sr and those of the hybrids are shown in Fig. 4A–E. Fur is made of flattened sticks with length ranging from one micron to a few tens of micron (Fig. 4A). HAP-Pure occurs in the form of dense aggregates of small, rounded particles (Fig. 4B) and the same is true for HAP-Sr (Fig. 4C), whose particles seem smaller and less compact with respect to the pure HAP. The adsorption measurements, in the BET portion, allowed determining a surface area and pore volume of 357 m2 g−1 and 0.24 cm3 g−1 for HAP-Sr with respect to 143 m2 g−1 and 0.08 cm3 g−1 for HAP-Pure. The areas are very high, if compared to analogous samples prepared by similar synthesis routes [26, 27], suggesting the formation of highly microporous hydroxyapatites. An effect of Sr substitution is obviously evident on both surface area and pore volume, due to the same kind of synthesis, probably due to the lower degree of crystallinity of HAP-Sr with respect to the HAP-Pure, and to the lower crystallite sizes values, as can be deduced from the peaks’ broadening of the diffraction patterns (Fig. 1). These features could be positive for the subsequent drug loading, which could be, in principle, expected to be higher for HAP-Sr sample.
The hybrids’ morphologies resemble those of the corresponding HAP samples (Fig. 4D, E): aggregates of small, rounded particles are again well evident, confirming that the adsorption of furosemide does not change the external morphology. No particles attributable to pure drug are visible.
The EDS microanalysis, combined with SEM, is a widespread tool to check the homogeneity of cations distribution, and therefore, in our case, to support the hybrids formation by analysing the presence of the characteristic elements of HAP (Ca and P ions, and Sr substituent) and Furosemide (Cl and/or S ions). The elemental maps obtained for the hybrid compounds show a good homogeneous distribution of all the elements and show that Cl and S (not shown) ions are located in the same points of the powders, where also Ca and P are mainly concentrated (Fig. 5). This observation obviously supports the hybrids’ formation. For all the samples, the EDS spectra allowed to determine the chemical compositions, which were found in good agreement with the expected stoichiometries. The Ca/P ratios are near to the ideal 1.67 value for HAP-Pure and HAP-Pure-Fur samples and to the expected value of 1 for the Sr substituted samples, having the stoichiometry Ca6Sr4(PO4)6(OH)2. In addition, the Sr substituent is present in the expected amount (Table 1). The peaks of S and Cl, both elements present in the Furosemide molecule, confirm the existence of the drug in the analysed samples. Their amount, in both the hybrids, is the same, due to the presence of 1 Cl and 1 S atoms in the furosemide molecule. From the atomic ratios (Table 1) we can calculate a drug loading of about 9.1 wt% and 8.3 wt% for HAP-Sr-Fur and HAP-Pure-Fur respectively. These data will be compared with the amount of drug loading determined by UV–Vis spectroscopy (see par. 3.2).
3.2 Pharmaceutical Characterization
The drug loading was determined to be 8.2 ± 1.0% for HAP-Sr-Fur and 7.2 ± 0.8% for HAP-Pure-Fur and these values are in good agreement with those obtained from the EDS microanalyses (Table 1).
From the solubility experiment, it was possible to determine that, for both hybrids, the Fur solubility is greater than four times the dose (corresponding to 100 mg L−1), and much higher than the solubility of Fur alone (6 mg L−1) [9].
The contact angles measured for HAP-Pure-Fur and HAP-Sr-Fur in the different fluids show an increased wetting ability compared to Fur alone. This effect is immediately evident at 1 s after deposition (Fig. 6), but increases more significantly over time (Fig. 7) in all the media considered. HAP-Sr-Fur starts from values of 45–55° θ, which are lower compared to those of HAP-Pure-Fur, 55–65° θ, and both are at least half the values obtained from Fur alone. Moreover, the contact angles of the two hybrids decrease quickly with time to 10–20° θ in few seconds, showing a fast wetting ability, also explaining their more rapid dissolution behaviour (see Fig. 8). On the other side, the contact angle of the drug remains constant throughout all the experiment (300 s) (Fig. 7).
The dissolution tests (Fig. 8) confirm the improved performances of the two hybrid compounds in comparison with the drug alone in all the fluids considered. Fur is quite insoluble at pH = 1 (simulating fasted gastric environment), but even in this condition HAP-Pure-Fur and HAP-Sr-Fur showed a very fast dissolution rate of the drug, reaching 90% of the dose dissolved in about 120 min. Even more evident is the improvement of the drug dissolution rate from the two hybrids in the fluid prescribed by the US Pharmacopoeia, pH = 5.8 buffer, in which the dose is completely delivered in less than 20 min. Only in the unbuffered condition (deionised water) a slight difference in the dissolution profile between HAP-Pure-Fur and HAP-Sr-Fur can be noticed, probably due to the faster wettability of this last compound. In any cases, both hybrids are able to greatly improve the Fur dissolution rate even in the neutral condition compared to the drug alone.
Our results represent a great improvement on the state of the art of furosemide drug delivery systems. In fact, our dissolution profiles in acidic medium (pH = 1) are similar or even better than those reported for inorganic–organic hybrids based on silica particles [13,14,15], which however do not report tests in the conditions suggested by Pharmacopoeia (pH = 5.8).
Furosemide, as already discussed, is characterized by a considerable variability in the intensity of the effect, due to its low solubility linked to the medium pH and to a very variable oral bioavailability. For this reason, the enhanced dissolution rate of Fur in the different fluids is a great advantage especially in the therapy of critical diseases, when the effect should be reached as soon as possible.
4 Conclusions
The successful loading of Furosemide onto HAP surfaces was proved with the combined use of different experimental techniques. The Sr doping greatly increased the surface area of the HAP, but only slightly increased the drug loading with respect to pure HAP. This can be due to the formation of micro-porosity that does not fit well with Furosemide sizes. However, the new hybrids show greatly improved pharmaceutical properties with respect to Furosemide alone in terms of solubility, wettability and dissolution rate in all the fluids considered to simulate the gastro-intestinal environment. These characteristics could enhance the bioavailability of the drug and promote a quick appearance of the therapeutic effect. This is particularly useful in critical pathologies when it is necessary to rapidly decrease the blood pressure of the patient. Notwithstanding the obtained drug loading is fairly low, the adsorption of drugs onto HAP surface was however revealed a winning strategy. Through the optimization of HAP surfaces and porosity, highly performing systems based on HAP hosts could be developed in future for different poorly soluble drugs.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Change history
20 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
R. Saravanakumar, K. Muthukumaran, C. Sivasankari, N. Sathiyapriya, K. Sakthipandi, Water Air Soil Pollut. 233, 53 (2022)
Z. Yang, Y. Zhong, X. Zhou, W. Zhang, Y. Yin, W. Fang, H. Xue, J. Food Measur. Character. (2022). https://doi.org/10.1007/s11694-021-01270-5
C.N.R. Rao, A.K. Cheetham, A. Thirumurugan, J. Phys. Condens. Matter 20, 083202 (2008)
S. Inocencio, T. Cordeiro, I. Matos, F. Danede, J.C. Sotomayor, I.M. Fonseca, N.T. Correia, M.C. Corvo, M. Dionísio, Micropor. Mesopor. Mater. 310, 110541 (2021)
E. Sayed, C. Karavasili, K. Ruparelia, R. Haj-Ahmad, G. Charalambopoulou, T. Steriotis, D. Giasafaki, P. Cox, N. Singh, L.-P.N. Giassafaki, A. Mpenekou, C.K. Markopoulou, I.S. Vizirianakis, M.-W. Chang, D.G. Fatouros, Z. Ahmada, J. Controlled Release 278, 142 (2018)
M. Bini, F. Monteforte, I. Quinzeni, V. Friuli, L. Maggi, G. Bruni, J. Solid State Chem. 272, 131 (2019)
D. Capsoni, I. Quinzeni, G. Bruni, V. Friuli, L. Maggi, M. Bini, J. Pharm. Sci. 107, 267 (2018)
M. Guagliano, F. Monteforte, G. Bruni, V. Friuli, L. Maggi, I. Quinzeni, M. Bini, Appl. Clay Sci. 198, 105826 (2020)
C.T. Supuran, Current Pharm. Design 14, 641 (2008)
N.J. Babu, S. Cherukuvada, R. Thakuria, A. Nangia, Cryst. Growth Des 10, 1979 (2010)
M.M. Devilliers, J.G. Van Der Watt, A.P. Lotter, W. Liebenberg, T.G. Dekker, Drug Dev. Ind. Pharm. 21, 1975 (1995)
F. Carta, C.T. Supuran, Expert Opin. Therap. Patients 23, 681 (2013)
V. Ambrogi, L. Perioli, C. Pagano, F. Marmottini, M. Ricci, A. Sagnella, C. Rossi, Eur. J. Pharm. Sci. 46, 43 (2012)
V. Ambrogi, L. Perioli, C. Pagano, L. Latterini, F. Marmottini, M. Ricci, C. Rossi, Micropor. Mesopor. Mater. 147, 343 (2012)
A. Zvonar, K. Berginc, A. Kristl, M. Gasperlin, Int. J. Pharm. 388, 151 (2010)
K.U.B. Rao, S. Gangavaram, N.R. Goud, S. Cherukuvada, S. Raghavender, A. Nangia, S.G. Manjunatha, S. Nambiar, S. Pal, Cryst. Eng. Commum. 16, 4842 (2014)
N.R. Goud, S. Gangavaram, K. Suresh, S. Pal, S.G. Manjunatha, S. Nambiar, A. Nangia, J. Pharm. Sci. 101, 664 (2012)
B.I. Harriss, L. Vella-Zarb, C. Wilson, I. Radosavljevic Evans, Cryst. Growth Des. 14, 783 (2014)
V.K. Srirambhatla, A. Kraft, S. Watt, A.V. Powell, Cryst. Eng. Comm. 16, 9979 (2014)
T. Ueto, N. Takata, N. Muroyama, A. Nedu, A. Sasaki, S. Tanida, K. Terada, Cryst. Growth Des. 12, 485 (2012)
V. Bystrov, E. Paramonova, L. Avakyan, J. Coutinho, N. Bulina, Nanomaterials 11, 2752 (2021)
A. García, M.V. Cabañas, J. Peña, S. Sánchez-Salcedo, Pharmaceutics 13, 1981 (2021)
R. Rial, M. González-Durruthy, Z. Liu, J.M. Ruso, Molecules 26, 3190 (2021)
S. Mofakhami, E. Salahinejad, J. Controlled Release 338, 527 (2021)
S.L. Ochoa, W. Ortega-Lara, C.E. Guerrero-Beltrán, Pharmaceutics 13, 1642 (2021)
X. Ding, J. Zheng, F. Ju, L. Wang, J. Kong, J. Feng, T. Liu, Ceram. Int. 47, 34836 (2021)
R.C.R. dos Apostolos, G.F. Andrade, W.M. da Silva, D. de Assis Gomes, M.C. de Miranda, E.M.B. de Sousa, Int. J. Appl. Ceram. Technol. 16, 1836 (2019)
S. Saber-Samandari, S. Saber-Samandari, M. Gazi, F.C. Cebeci, E. Talasaz, J. Macromol, Sci. Part A 50, 1133 (2013)
A. Alves-Barbosa, S. Alves-Júnior, R.L. Mendes, R.S. de Lima, A. de Vasconcelos Ferraz, Mater. Sci. Eng. C 116, 111227 (2020)
Y. Xu, L. An, L. Chen, L. Cao, D. Zeng, G. Wang, Mater. Chem. Phys. 214, 359 (2018)
M. Vila, A. García, A. Girotti, M. Alonso, J.C. Rodríguez-Cabello, A. González-Vázquez, J.A. Planell, E. Engel, J. Buján, N. García-Honduvilla, M. Vallet-Regí, Acta Biomater. 45, 349 (2016)
Furosemide tablets/official monographs. In: The United States Pharmacopeia (USP41-NF36). Rockville, MD: United States Pharmacopeial Convention, Inc; 2018 pp.1894–95
Reagents. Solutions/Buffer solutions. The United States Pharmacopeia (USP40 -NF35). United States Pharmacopeial Convention, Inc., Rockville, MD; 2017. pp. 2409–2411.
Y. Matsuda, E. Tatsumi, Int. J. Pharm. 60, 11 (1990)
C. Doherty, P. York, Int. J. of Pharmaceutics 47, 141 (1988)
R.D. Shannon, Acta Crystallogr. A 32, 751 (1976)
N. Lenin, K. Sakthipandi, R. Rajesh-Kanna, G. Rajkumar, Ceram. Int. 44, 21866 (2018)
S. Ferrari, M.C. Mozzati, M. Lantieri, G. Spina, D. Capsoni, M. Bini, Sci. Rep. 6, 27896 (2016)
B.O. Fowler, Inorg. Chem. 13, 194 (1974)
H. Wang, L. Zhai, Y. Li, T. Shi, Mat. Res. Bull. 43, 1607 (2008)
Acknowledgements
The authors are grateful to Prof. Vittorio Berbenni for the surface area measurements.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
MLR and AR: Investigation, Methodology; GB, VF, LM: Investigation, Validation, Formal analysis, Writing-Review & Editing; MB: Conceptualization, Supervision, Writing—Original Draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Rocca, M., Rinaldi, A., Bruni, G. et al. New Emerging Inorganic–Organic Systems for Drug-Delivery: Hydroxyapatite@Furosemide Hybrids. J Inorg Organomet Polym 32, 2249–2259 (2022). https://doi.org/10.1007/s10904-022-02302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02302-3