Abstract
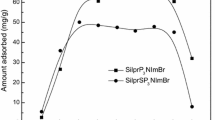
Due to the danger of heavy metals such as Pb(II), Cu(II), Co(II), and Ni(II) ions to the environment and humans, the world needs to develop new effective adsorbents to get rid of them. Many Schiff bases can form chelates with most heavy metal ions. Hence, loading them on supports like silicon oxide as new composites help to solve the pollution problem. So, in this work, a new composite based on the formation of Schiff base on silica nanoparticles was facilely synthesized. (3-aminopropyl)trimethoxysilane was used to modify silica nanoparticles with silanol groups (Si–OH). Then, the modified silica was then combined with 4,6-diacetylresorcinol to create a new Schiff base/silica composite. XRD, FE-SEM, FT-IR, CHN analyzer, and N2 adsorption/desorption analyzer were used to characterize the synthesized composite. The formation of the Schiff base results in a significant drop in the intensity of the composite XRD peak at 2Ɵ = 21.9°. In addition, the FT-IR bands at 3443 and 1606 cm−1 are due to the stretching and bending vibrations of OH and/or C=N, respectively. The FE-SEM images confirmed that the silica has uneven forms while the composite has a flaky surface due to the formation of the Schiff base. According to an elemental analysis of the composite, the percentages of C, H, and N are 15.26, 3.24, and 1.65%, respectively. The produced Schiff base restricts the pores of silica and hence the composite BET surface area and total pore volume were lowered. The synthesized composite was used to remove Pb(II), Cu(II), Co(II), and Ni(II) ions from aqueous solutions with high efficiency. The maximum uptake capacity of the composite toward Pb(II), Cu(II), Co(II), or Ni(II) ions is 107.066, 89.767, 80.580, and 70.972 mg/g, respectively. The adsorption processes of the investigated metal ions were chemical, spontaneous, and well fitted with the Langmuir equilibrium isotherm and pseudo-second-order kinetic model. The synthesized composite can be successfully regenerated and utilized various times in the removal of investigated metal ions from aqueous solutions.














Similar content being viewed by others
References
M. Sultana, M.H. Rownok, M. Sabrin, M.H. Rahaman, S.M.N. Alam, A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 6, 100382 (2022)
J. Li, Z. Lei Yang, T. Ding, Y.J. Song, H.C. Li, D. Qiang Li, S. Chen, F. Xu, The role of surface functional groups of pectin and pectin-based materials on the adsorption of heavy metal ions and dyes. Carbohydr. Polym. 276, 118789 (2022)
U.O. Aigbe, K.E. Ukhurebor, R.B. Onyancha, O.A. Osibote, H. Darmokoesoemo, H.S. Kusuma, Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J. Mater. Res. Technol. 14, 2751–2774 (2021)
E.A. Abdelrahman, R.M. Hegazey, R.E. El-Azabawy, Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol. 8, 5301–5313 (2019)
R.M. Hegazey, E.A. Abdelrahman, Y.H. Kotp, A.M. Hameed, A. Subaihi, Facile fabrication of hematite nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of rhodamine B dye. J. Mater. Res. Technol. 9, 1652–1661 (2020)
N.H. Abdullah, K. Shameli, E.C. Abdullah, L.C. Abdullah, Solid matrices for fabrication of magnetic iron oxide nanocomposites: synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 162, 538–568 (2019)
M.T. ALSamman, J. Sánchez, Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arab. J. Chem. 14, 102455 (2021)
S. Dubey, M. Shri, A. Gupta, V. Rani, D. Chakrabarty, Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 16, 1169–1192 (2018)
S.R. Mallampati, Y. Mitoma, T. Okuda, S. Sakita, M. Kakeda, Total immobilization of soil heavy metals with nano-Fe/Ca/CaO dispersion mixtures. Environ. Chem. Lett. 11, 119–125 (2013)
E.R. Sumner, A. Shanmuganathan, T.C. Sideri, S.A. Willets, J.E. Houghton, S.V. Avery, Oxidative protein damage causes chromium toxicity in yeast. Microbiology 151, 1939–1948 (2005)
M. Słota, M. Wąsik, T. Stołtny, A. Machoń-Grecka, S. Kasperczyk, Effects of environmental and occupational lead toxicity and its association with iron metabolism. Toxicol. Appl. Pharmacol. 434, 115794 (2022)
G. Pascual, D. Sano, T. Sakamaki, M. Akiba, O. Nishimura, The water temperature changes the effect of pH on copper toxicity to the green microalgae Raphidocelis subcapitata. Chemosphere 291, 133110 (2022)
G.K. Macoustra, D.F. Jolley, J.L. Stauber, D.J. Koppel, A. Holland, Speciation of nickel and its toxicity to Chlorella sp. in the presence of three distinct dissolved organic matter (DOM). Chemosphere 273, 128454 (2021)
V. Verougstraete, R. Danzeisen, V. Viegas, P. Marsh, A. Oller, A tiered approach to investigate the inhalation toxicity of cobalt substances. Tier 1: bioaccessibility testing. Regul. Toxicol. Pharmacol. 129, 105124 (2022)
A.K. Sinha, V. Matey, T. Giblen, R. Blust, G. De Boeck, Gill remodeling in three freshwater teleosts in response to high environmental ammonia. Aquat. Toxicol. 155, 166–180 (2014)
L.A. Malik, A. Bashir, A. Qureashi, A.H. Pandith, Detection and removal of heavy metal ions: a review. Environ. Chem. Lett. 17, 1495–1521 (2019)
E.A. Abdelrahman, Y.G. Abou El-Reash, H.M. Youssef, Y.H. Kotp, R.M. Hegazey, Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 401, 123813 (2021)
M.A. Barakat, New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4, 361–377 (2011)
Y. Sun, S. Zhou, W. Sun, S. Zhu, H. Zheng, Flocculation activity and evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Sep. Purif. Technol. 241, 116737 (2020)
W. Zeng, W. Guo, B. Li, Z. Wei, D.D. Dionysiou, R. Xiao, Kinetics and mechanistic aspects of removal of heavy metal through gas-liquid sulfide precipitation: a computational and experimental study. J. Hazard. Mater. 408, 124868 (2021)
Y. Ding, J. Wu, J. Wang, J. Wang, J. Ye, F. Liu, Superhydrophilic carbonaceous-silver nanofibrous membrane for complex oil/water separation and removal of heavy metal ions, organic dyes and bacteria. J. Memb. Sci. 614, 118491 (2020)
P. Wu, X. Wu, Y. Wang, H. Xu, G. Owens, A biomimetic interfacial solar evaporator for heavy metal soil remediation. Chem. Eng. J. 435, 134793 (2022)
S. Pan, J. Shen, Z. Deng, X. Zhang, B. Pan, Metastable nano-zirconium phosphate inside gel-type ion exchanger for enhanced removal of heavy metals. J. Hazard. Mater. 423, 127158 (2022)
E.A. Abdelrahman, A. Alharbi, A. Subaihi, A.M. Hameed, M.A. Almutairi, F.K. Algethami, H.M. Youssef, Facile fabrication of novel analcime/sodium aluminum silicate hydrate and zeolite Y/faujasite mesoporous nanocomposites for efficient removal of Cu(II) and Pb(II) ions from aqueous media. J. Mater. Res. Technol. 9, 7900–7914 (2020)
A.M. Hameed, A. Alharbi, E.A. Abdelrahman, E.M. Mabrouk, R.M. Hegazey, F.K. Algethami, Y.O. Al-Ghamdi, H.M. Youssef, Facile hydrothermal fabrication of analcime and zeolite X for efficient removal of Cd(II) ions from aqueous media and polluted water. J. Inorg. Organomet. Polym. Mater. 30, 4117–4128 (2020)
E.A. Abdelrahman, R.M. Hegazey, Utilization of waste aluminum cans in the fabrication of hydroxysodalite nanoparticles and their chitosan biopolymer composites for the removal of Ni(II) and Pb(II) ions from aqueous solutions: kinetic, equilibrium, and reusability studies. Microchem. J. 145, 18–25 (2019)
E.A. Abdelrahman, R.M. Hegazey, Exploitation of Egyptian insecticide cans in the fabrication of Si/Fe nanostructures and their chitosan polymer composites for the removal of Ni(II), Cu(II), and Zn(II) ions from aqueous solutions. Compos. Part B Eng. 166, 382–400 (2019)
M.E. Khalifa, E.A. Abdelrahman, M.M. Hassanien, W.A. Ibrahim, Application of mesoporous silica nanoparticles modified with dibenzoylmethane as a novel composite for efficient removal of Cd(II), Hg(II), and Cu(II) ions from aqueous media. J. Inorg. Organomet. Polym. Mater. 30, 2182–2196 (2020)
E.A. Abdelrahman, A. Subaihi, Application of geopolymers modified with chitosan as novel composites for efficient removal of Hg(II), Cd(II), and Pb(II) ions from aqueous media. J. Inorg. Organomet. Polym. Mater. 30, 2440–2463 (2020)
M. Adibmehr, H. Faghihian, Magnetized activated carbon prepared by oak shell biowaste and modified with nickel hexacyanoferrate for selective removal of cesium. J. Inorg. Organomet. Polym. Mater. 29, 1941–1955 (2019)
I.O. Ali, S.M. El-Sheikh, T.M. Salama, E.K. Abdel-Khalek, M.S. Thabet, M.F. Bakr, M.H. Fodial, Novel composites of multifunctional NaP zeolite/graphene oxide for highly efficient removal of Fe(III) from aqueous solution. J. Inorg. Organomet. Polym. Mater. 31, 577–590 (2021)
M. Dinari, N. Roghani, Calcium iron layered double hydroxide/poly(vinyl chloride) nanocomposites: synthesis, characterization and Cd2+ removal behavior. J. Inorg. Organomet. Polym. Mater. 30, 808–819 (2020)
A.T. Hoang, S. Nižetić, C.K. Cheng, R. Luque, S. Thomas, T.L. Banh, V.V. Pham, X.P. Nguyen, Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: a comprehensive review. Chemosphere 287, 131959 (2022)
A.M. Naglah, M.A. Al-Omar, A.A. Almehizia, H.M. AlKahtani, A.J. Obaidullah, M.A. Bhat, N.S. Al-Shakliah, Application of nanosized zeolite X modified with glutamic acid as a novel composite for the efficient removal of Co(II) ions from aqueous media. J. Inorg. Organomet. Polym. Mater. 31, 2105–2115 (2021)
S. Rahpeima, V. Javanbakht, J. Esmaili, Synthesis and characterization of activated carbon/maghemite/starch magnetic bionanocomposite and its application for permanganate removal from aqueous solution. J. Inorg. Organomet. Polym. Mater. 28, 195–211 (2018)
H.S. Far, M. Hasanzadeh, M. Najafi, T.R.M. Nezhad, M. Rabbani, Efficient removal of Pb(II) and Co(II) ions from aqueous solution with a chromium-based metal-organic framework/activated carbon composites. Ind. Eng. Chem. Res. 60, 4332–4341 (2021)
H.S. Far, M. Hasanzadeh, M.S. Nashtaei, M. Rabbani, Fast and efficient adsorption of palladium from aqueous solution by magnetic metal–organic framework nanocomposite modified with poly(propylene imine ) dendrimer. Environ. Sci. Pollut. Res. 28, 62474–62486 (2021)
S. Xue, H. Xie, H. Ping, Q. Li, B. Su, Z. Fu, Induced transformation of amorphous silica to cristobalite on bacterial surface. RSC Adv. 88, 1–11 (2015)
V. Correcher, J. Garcia-Guinea, M.A. Bustillo, R. Garcia, Study of the thermoluminescence emission of a natural α-cristobalite. Radiat. Eff. Defects Solids 164, 59–67 (2009)
Z.P. Xu, P.S. Braterman, High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J. Mater. Chem. 13, 268–273 (2003)
A.E. Shalapy, Y.G. Abou El-Reash, E.A. Abdelrahman, M.E. Khalifa, Facile synthesis and characterisation of novel Sn/Si mixtures for the efficient removal of methylene blue and crystal violet dyes from aqueous media. Int. J. Environ. Anal. Chem. (2021). https://doi.org/10.1080/03067319.2021.1986036
R.K. Shah, A.M. Naglah, M.A. Al-Omar, A.A. Almehizia, S. AlReshaidan, A. Subaihi, A. Alharbi, A.M. Hameed, J. Alkabli, M.E. Fetoh, A.A. Alluhaybi, A.S. Al-Wasidi, T.S. Alraddadi, H.M. Youssef, Efficient removal of Ni(II) ions from aqueous solutions using analcime modified with dimethylglyoxime composite. Arab. J. Chem. 14, 103197 (2021)
S.N. Adamovich, E.G. Filatova, Y.N. Pozhidaev, I.A. Ushakov, A.D. Chugunov, E.N. Oborina, I.B. Rozentsveig, F. Verpoort, Natural zeolite modified with 4-(3-triethoxysilylpropyl) thiosemicarbazide as an effective adsorbent for Cu(II), Co(II) and Ni(II). J. Taiwan Inst. Chem. Eng. 129, 396–409 (2021)
C. Chen, H. Liu, T. Chen, D. Chen, R.L. Frost, An insight into the removal of Pb(II), Cu(II), Co(II), Cd(II), Zn(II), Ag(I), Hg(I), Cr(VI) by Na(I)-montmorillonite and Ca(II)-montmorillonite. Appl. Clay Sci. 118, 239–247 (2015)
I.M. Kenawy, M.A.H. Hafez, M.A. Ismail, M.A. Hashem, Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int. J. Biol. Macromol. 107, 1538–1549 (2018)
M. Osińska, Removal of lead(II), copper(II), cobalt(II) and nickel(II) ions from aqueous solutions using carbon gels. J. Sol-Gel Sci. Technol. 81, 678–692 (2017)
A. Uheida, G. Salazar-Alvarez, E. Björkman, Z. Yu, M. Muhammed, Fe3O4 and γ-Fe2O3 nanoparticles for the adsorption of Co2+ from aqueous solution. J. Colloid Interface Sci. 298, 501–507 (2006)
B. Körpınar, A. Erdem Yayayürük, O. Yayayürük, H. Akat, Thiol–ended polycaprolactone: synthesis, preparation and use in Pb(II) and Cd(II) removal from water samples. Mater. Today Commun. 29, 102908 (2021)
Acknowledgements
The authors are grateful to Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia for funding this work through Researches Supporting Project number (PNURSP2022R35). The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Funding
This work was funded by Princess Nourah Bint Abdulrahman University & King Saud University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interest for this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Wasidi, A.S., Naglah, A.M., Saad, F.A. et al. Modification of Silica Nanoparticles with 4,6-Diacetylresorcinol as a Novel Composite for the Efficient Removal of Pb(II), Cu(II), Co(II), and Ni(II) Ions from Aqueous Media. J Inorg Organomet Polym 32, 2332–2344 (2022). https://doi.org/10.1007/s10904-022-02282-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02282-4