Abstract
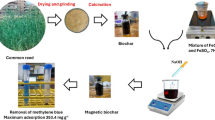
The activated carbon synthesized by biowaste materials was magnetized by Fe3O4 nanoparticles and then impregnated with nickel hexacyanoferrate. The magnetize composite was identified by various methods including; XRD, FT-IR, TG, VSM and SEM technique and then employed for separation of cesium from aquatic systems. The adsorption capacity was optimized by evaluating different experimental variables including time, pH, dose, and concentration, and the maximal uptake of 135.28 mg g−1 was obtained. Evaluation of adsorbent selectivity was performed with co-existing cations including Mg2+, Ca2+, Na+, K+ and NH4+. The results revealed that removal process was endothermic, spontaneous, with fast kinetic. The adsorption data was evaluated using Langmuir, Freundlich, Sips, and Redlich-Peterson isotherm models. The data was well described by the Langmuir model. The pseudo first-order, pseudo second-order, Intra-particle diffusion equation and Elovich kinetic models were used to evaluate the kinetic data. The used sorbent regenerated by use of nitric acid solution retained most of its initial capacity.













Similar content being viewed by others
References
M.M. Abd El-Latif, M.F. Elkady, Equilibrium isotherms for harmful ions sorption using nano zirconium vanadate ion exchanger. Desalination 255, 21–43 (2010)
M. Adibmehr, H. Faghihian, Magnetization and functionalization of activated carbon prepared by oak shell biowaste for removal of Pb2+ from aqueous solutions. Chem. Eng. J. 205, 519–532 (2018)
M. Adibmehr, H. Faghihian, Novel magnetic biorbent prepared by oak shell waste material as an efficient adsorbent for consecutive removal of Pb2+, Ag+, Ba2+, Sr2+ and chromate from aqueous solutions. C. R. Chim. 21, 840–853 (2018)
Sh Amanipour, H. Faghihian, Potassium hexacyanoferrate-clinoptilolite adsorbent for removal of Cs+ and Sr2+ from aqueous solutions. Int. J. Environ. Stud. 74, 86–104 (2017)
S.V. Avery, Cesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Chem. Technol. Biotechnol. 62, 76–84 (1995)
M.R. Awual, T. Yaita, T. Taguchi, H. Shiwaku, Sh Suzuki et al., Selective cesium removal from radioactive liquid waste by crown ether immobilized new class conjugate adsorbent. J. Hazard. Mater. 278, 227–235 (2014)
E. Bascetin, H. Haznedaroglu, A.Y. Erkol, The adsorption behavior of cesium on silica gel. Appl. Radiat. Isot. 59, 5–9 (2003)
M. Caccin, F. Giacobbo, M. Da Ros, L. Besozzi, M. Mariani, Adsorption of uranium, cesium and strontium onto coconut shell activated carbon. J. Radioanal. Nucl. Chem. 297(1), 9–18 (2013)
M. Chahud, F.M.A.S.C. Ilho, N.S. Fernandes, M.I. Shiro, A thermal analysis study of dithizone and dithizonates of mercury, silver and bismuth. Eclética Química 25, 1–6 (2000)
C.Y. Chang, L.K. Chau, W.P. Hu, C.Y. Wang, J.H. Liao, Nickel hexacyanoferrate multilayers on functionalized mesoporous silica supports for selective sorption and sensing of cesium. Microporous Mesoporous Mater. 109, 505–512 (2008)
R. Chen, H. Tanaka, T. Kawamoto, M. Asai, C. Fukushima, H. Na et al., Selective removal of cesium ions from wastewater using copper hexacyanoferrate nano films in an electrochemical system. Electron. Chim. Acta 87, 119–125 (2013)
W. Chen, L. Pan, L. Chen, Q. Wang, Ch. Yan, Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: Iron loading, kinetics and pathway. R. Soc. Chem. Adv. 4, 46689–46696 (2014)
R. Cortés-Martínez, M.T. Olguín, M. Solache-Ríos, Cesium sorption by clinoptilolite-rich tuffs in batch and fixed-bed systems. Desalination 258, 164–170 (2010)
M. Dashtinejad, M. Samadfam, J. Fasihi, F. Grayeli-Fumeshkenar, H. Sepehrian, Synthesis, characterization, and cesium sorption performance of potassium nickel hexacyanoferrate-loaded granular activated carbon. Part. Sci. Technol. 32, 348–354 (2014)
C. Delchet, A. Tokarev, X. Dumail, G. Toquer, Y. Barre, Y. Guari et al., Extraction of radioactive cesium using innovative functionalized porous materials. R. Soc. Chem. Adv. 2, 5707–5716 (2012)
D. Ding, Y. Zhao, S. Yang, W. Shi, Z. Zhang, Z. Lei, Y. Yang, Adsorption of cesium from aqueous solution using agricultural residue e Walnut shell: Equilibrium, kinetic and thermodynamic modeling studies. Water Res. 41, 2563–2571 (2013)
D. Ding, Z. Lei, Y. Yang, Ch. Feng, Z. Zhang, Selective removal of cesium from aqueous solutions with nickel (II) hexacyanoferrate (III) functionalized agricultural residue–walnut shell. J. Hazard. Mater. 270, 187–195 (2014)
H.M.H. Gad, H.A. Elsana fi nia, M.M.S. Ali, Y.F. Lasheen, M.G. Abdelwahed, Factors affecting the sorption behavior of Cs+ and Sr2+ using biosorbent material. Russ. J. Appl. Chem. 89(6), 988–999 (2016)
Y.P.K. Gallagher, B. Precott, Further studies of the thermal decomposition of Europium hexacyanoferrate(II1) and ammonium europium hexacyanoferrate(I1). Inorg. Chem. 9, 2510–2512 (1970)
N. Genevois, N. Villandier, V. Chaleix, E. Poli, L. Jauberty, V. Gloaguen, Removal of cesium ion from contaminated water: Improvement of Douglas fir bark biosorption by a combination of nickel hexacyanoferrate impregnation and TEMPO oxidation. Ecol. Eng. 100, 186–193 (2017)
F. Han, G.H. Zhang, P. Gu, Removal of cesium from simulated liquid waste with countercurrent two-stage adsorption followed by microfiltration. J. Hazard. Mater. 225, 107–113 (2012)
S. Hashemian, H. Saffari, S. Ragabion, Adsorption of Cobalt(II) from aqueous solutions by Fe3O4/bentonite nanocomposite. Water Air Soil Pollut. 226, 2212–2222 (2015)
G. Huang, J.X. Shi, T.A. Langrish, Removal of Cr(VI) from aqueous solution using activated carbon modified with nitric acid. Chem. Eng. J. 152(2), 434–439 (2009)
R. Jalali-Rad, H. Ghafourian, Y. Asef, S.T. Dalir, M.H. Sahafipour, B.M. Gharanjik, Biosorption of cesium by native and chemically modified biomass of marine algae: introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. B 116, 125–134 (2004)
W. Jin, A. Toutianoush, M. Pyrasch, J. Schnepf, H. Gottschalk, W. Rammensee, B. Tieke, Self-assembled films of prussian blue and analogues: structure and morphology, elemental composition, film growth, and nanosieving of ions. J. Phys. Chem. B 107, 12062–12070 (2003)
X. Jin, L. Huang, Sh Yu, M. Ye, J. Yuana, J. Shen et al., Selective electrochemical removal of cesium ion based on nickel hexacyanoferrate/reduced graphene oxide hybrids. Sep. Purif. Technol. 209, 65–72 (2019)
R. Ishihara, K. Fujiwara, T. Harayama, Y. Okamura, Sh Uchyama, M. Baba et al., Removal of cesium using cobalt ferrocyanide impregnated polymer-chain-grafted fibers. J. Nucl. Sci. Technol. 48(10), 1281–1284 (2011)
Y. Lin, G.E. Fryxell, H. Wu, M. Engelhard, Selective sorption of cesium using self-assembled monolayers on mesoporous supports. Environ. Sci. Technol. 35, 3962–3966 (2001)
H. Kazemian, H. Zakeri, M.S. Rabbani, Cs and Sr removal from solution using potassium nickel hexacyanoferrate impregnated zeolites. J. Radioanal. Nucl. Chem. 268(2), 231–236 (2006)
Sh Khandaker, T. Kuba, S. Kamida, Y. Uchikawa, Adsorption of cesium from aqueous solution by raw and concentrated nitric acid-modified bamboo charcoal authors. J. Environ. Chem. Eng. 5, 1456–1464 (2017)
Y.P. Kumar, P. King, V.S.R.K. Prasad, Equilibrium and kinetic studies for the biosorption system of copper (II) ion from aqueous solution using 20 Tectona grandis Lf leaves powder. J. Hazard. Mater. 137(2), 1211–1217 (2006)
C.L. Lalhmunsiama, D. Tiwari, S.M. Lee, Immobilized nickel hexacyanoferrate on activated carbons for efficient attenuation of radio toxic Cs(I) from aqueous solutions. Appl. Surf. Sci. 321, 275–282 (2014)
F. Lasheen, H.M.H. Gad, T.S. El-Zakla, Efficiency of locally prepared activated carbon in the preconcentration of barium-133 and radium-226 radionuclides in single and binary systems. Radiochemistry 55, 589–595 (2013)
A.Y. Lonin, V.V. Levenets, I.M. Neklyudov, A.O. Shchur, The usage of zeolites for dynamic sorption of cesium from waste waters of nuclear power plants. J. Radioanal. Nucl. Chem. 303, 831–836 (2015)
J. Mizera, G. Mizerová, V. Machovič, L. Borecká, Sorption of cesium, cobalt and europium on low-rank coal and chitosan. Water Res. 41(3), 620–626 (2007)
Sh Naeimi, H. Faghihian, Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep. Purif. Technol. 175, 255–265 (2017)
A. Nilchi, A. Khanchi, M. GhanadiMaragheh, The importance of cerium substituted phosphates as cation exchanger some unique properties and related application potentials. Talanta 56(3), 383–393 (2002)
A.E. Ofomaja, A. Pholosi, E.B. Naidoo, Kinetics and competitive modeling of cesium biosorption onto iron(III) hexacyanoferrate modified pine cone powder. Int. Biodeterior. Biodegradation 92, 71–78 (2014)
A.E. Ofomaja, A. Pholosi, E.B. Naidoo, Application of raw and modified pine biomass material for cesium removal from aqueous solution. Ecol. Eng. 82, 258–266 (2015)
P. Panneerselvam, N. Morad, K. Tan, Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 186, 160–168 (2011)
H. Parab, M. Sudersanan, Engineering a lignocellulosic biosorbente coir pith for removal of cesium from aqueous solutions: equilibrium and kinetic studies. Water Res. 44(3), 854–860 (2010)
D. Parajuli, A. Kitajima, A. Takahashi, H. Tanaka, H. Ogawa, Y. Hakuta et al., Application of Prussian blue nanoparticles for the radioactive Cs decontamination in Fukushima region. J. Environ. Radioact. 151, 233–237 (2016)
W. Plazinski, W. Rudzinski, Modeling the effect of surface heterogeneity in equilibrium of heavy metal ion biosorption by using the ion exchange model. Environ. Sci. Technol. 43(19), 7465–7471 (2009)
M. Poletto, J. Dettenborn, V. Pistor, M. Zeni, A. Zattera, Materials produced from plant biomass. Part I: evaluation of thermal stability and pyrolyses of wood. J. Mater. Res. 13, 375–389 (2010)
K. Rajczykowski, O. Sałasiń ska, K. Loska, Zinc removal from the aqueous solutions by the chemically modified biosorbents. Water Air Soil Pollut. 229, 1–7 (2018)
H. Rogers, J. Bowers, D. Gates-Anderson, An isotope dilution–precipitation process for removing radioactive cesium from waste water. J. Hazard. Mater. 243, 124–129 (2012)
R. Saberi, A. Nilchi, S. RasoliGarmarodi, R. Zarghami, Adsorption characteristic of 137Cs from aqueous solution using PAN-based sodium titanosilicate composite. J. Radioanal. Nucl. Chem. 284, 461–469 (2010)
R.R. Sheha, Synthesis and characterization of magnetic hexacyanoferrate (II) polymeric nanocomposite for separation of cesium from radioactive waste solutions. J. Colloid Sci. 388, 21–30 (2012)
K. Shiozaki, N. Shigemot, Comparative study of Cs-uptake performance by potassium iron(III) hexacyanoferrate(II) supported on wood chips and activated carbon. J. Chem. Eng. Jpn. 46, 601–608 (2013)
K. Subramanian, P.S. Kumar, I.P. Jayapa, N. Venkatesh, Characterization of lingo-cellulosic seed fibre from WrightiaTinctoria plant for textile applications- an exploratory investigation. Eur. Polymer J. 41, 853–866 (2005)
S. Vashnia, H. Tavakoli, R. Cheraghali, H. Sepehrian, Zinc hexacyanoferrate loaded mesoporous MCM-41 as a new adsorbent for cesium: equilibrium, kinetic and thermodynamic studies. Desalin Water Treat 55, 1220–1228 (2014)
T. Vincent, C. Vincent, E. Guibal, Immobilization of metal hexacyanoferrate ion- exchangers for the synthesis of metal ion sorbents-A mini-review. Molecules 20, 20582–20613 (2015)
A.K. Vipin, B. Hu, B. Fugetsu, Prussian blue caged in alginate/calcium beads as adsorbents for removal of cesium ions from contaminated water. J. Hazard. Mater. 258–259, 93–101 (2013)
K. Volchek, M.Y. Miah, W. Kuang, Z. DeMaleki, F.H. Tezel, Adsorption of cesium on cement mortar from aqueous solutions. J. Hazard. Mater. 194, 331–337 (2011)
L. Vrtoch, M. Pipı´sˇka, M. Hornı´k, J. Augustı´n, J. Lesny, Sorption of cesium from water solutions on potassium nickel hexacyanoferrate-modified Agaricus bisporus mushroom biomass. J. Radioanal. Nucl. Chem. 287, 853–862 (2011)
F.Y. Wang, H. Wang, J.W. Ma, Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent-Bamboo charcoal. J. Hazard. Mater. 177(1), 300–306 (2010)
Zh Wang, H. Guo, F.N. She, G. Yang, Y. Zhang, Y. Zeng et al., Biochar produced from oak sawdust by lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4 +), nitrate (NO3 −), and phosphate (PO −43 ). Chemosphere 119, 646–653 (2015)
H. Wang, X. Xu, Zh Ren, B. Gao, Removal of phosphate and chromium (VI) from liquids by amine crosslinked nano-Fe3O4 biosorbent derived from corn straw. R. Soc. Chem. Adv. 6, 47237–47248 (2016)
J. Wu, B. Li, J. Liao, Y. Feng, D. Zhang, J. Zhao et al., Behavior and analysis of cesium adsorption on montmorillonite mineral. J. Environ. Radioact. 100(10), 914–920 (2009)
L. Xiaona, L. Airong, L. Mingzhong, T. Xingjun, Equilibrium and kinetic studies of copper biosorption by dead Ceriporia lacerata biomass isolated from the litter of an invasive plant in China. J. Environ. Health Sci. Eng. 13, 37–44 (2015)
S. Yamada, K. Kuwabara, K. Koumoto, Characterization of Prussian Blue analogue: nanocrystalline nickel-iron cyanide. Mater. Sci. Eng. B 49, 89–94 (1997)
H.R. Yu, J.Q. Hu, Z. Liu, X.J. Ju, R. Xie, W. Wang et al., Ion recognizable hydrogels for efficient removal of cesium ions from aqueous environment. J. Hazard. Mater. 323, 632–640 (2016)
ShY Zhao, D. Keun Lee, Ch. Woo Kim, H. Gil Cha, Y. Hwan Kim, Y. Soo Kang, Synthesis of magnetic nanoparticles of Fe3O4 and CoFe2O4 and their surface modification by surfactant adsorption. Bull. Korean Chem. Soc. 27, 237–242 (2006)
Acknowledgments
This work was performed in the Islamic Azad University, Shahreza Branch. The authors wish to thanks the co-operation of the university.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adibmehr, M., Faghihian, H. Magnetized Activated Carbon Prepared by Oak Shell Biowaste and Modified with Nickel Hexacyanoferrate for Selective Removal of Cesium. J Inorg Organomet Polym 29, 1941–1955 (2019). https://doi.org/10.1007/s10904-019-01154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01154-8