Abstract
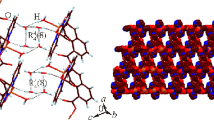
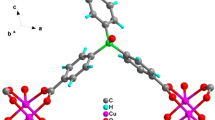
The coordination polymer [Cu2(TDPH)4(QNX)].DMF, (QNX = Quinoxaline; TDPH = 3,3-thiodipropionic acid), has been prepared by reaction of copper acetate, TDPH, and quinoxaline. The compound was characterized by elemental analysis, FTIR spectroscopy, and single-crystal X-ray diffraction. The crystal is monoclinic with a P21/n space group and dimensions of a = 12.889(3) Å, b = 14.983(4) Å, c = 14.091(3) Å, α = 90°, β = 90.200(11)°, γ = 90°, V = 2721.18 (2) Å3, Z = 4. The ligands are hexagonally coordinated to the Cu(II) centre in the form of Cu2O4N with one nitrogen atom from the quinoxaline ligand, and four oxygen atoms from four TDPH molecules in a monodentate fashion. The Cu–Cu bond length was 2.642(1) and 2.629(1) Å for the Cu1–Cu1 and Cu2–Cu2 bonds. The QNX ligand bridged the two copper atoms. The catalytic reduction of 4-nitrophenol to 4-aminophenol using NaBH4 in the presence of [Cu2(TDPH)4(QNX)].DMF, as catalyst was completed within 11 min. The 4-aminophenol product was confirmed using 1H NMR spectroscopy.














Similar content being viewed by others
References
A.C. Tella, S.O. Owalude, P.A. Ajibade, N. Simon, S.J. Olatunji, M.S.M. Abdelbaky, S. Garcia-Granda, J. Mol. Struct. 1125, 570–575 (2016)
A.C. Tella, S.O. Owalude, S.J. Olatunji, V.O. Adimula, S.E. Elaigwu, L.O. Alimi, P.A. Ajibade, O.S. Oluwafemi, J. Environ. Sci. 6, 264–275 (2018)
E.C. Constable, Chem. Soc. Rev. 42, 1637–1651 (2013)
A.C. Tella, S.O. Owalude, C.A. Ojekanmi, O.S. Oluwafemi, New J. Chem. 38, 4494–4500 (2014)
L. Ma, J.M. Falkowski, C. Abney, W. Lin, Nat. Chem. 2, 838–846 (2010)
D. Chen, Y. Cao, N. Chen, P. Feng, J. Inorg. Organomet. Polym. (2021). https://doi.org/10.1007/s10904-020-01832-y
P. Horcajada, C. Serre, G. Maurin, N.A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle, G. Férey, J. Am. Chem. Soc. 130, 6774–6780 (2008)
C. Zhu, Q. Xia, X. Chen, Y. Liu, X. Du, Y. Cui, ACS Catal. 6, 7590–7596 (2016)
H. Furukawa, K.E. Cordova, M. O’Keeffe, O.M. Yaghi, Science 341, 974–975 (2013)
L. Zhang, S. Qiu, G. Jiang, G. Jiang, R. Tang, Asian J. Org. Chem. 7, 165–170 (2018)
H.C. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112, 673–674 (2012)
G. Maurin, C. Serre, A. Cooper, G. Férey, Chem. Soc. Rev. 46, 3104–3107 (2017)
Y. Yang, X.X. Qi, H.R. Chen, X.Y. Wang, X.L. Yang, Y.Y. Tu, T. Zhou, T. Jiang, F. Wang, Z. Chen, Y.C. Ju, J. Inorg. Organomet. Polym. 30, 4289–4296 (2020)
W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III., M. Boscha, H.-C. Zhou, Chem. Soc. Rev. 43, 5561–5593 (2014)
J.X. Jiang, A. Trewin, D.J. Adams, A.I. Cooper, Chem. Sci. 2, 1777–1781 (2011)
G. Cheng, T. Hasell, A. Trewin, D.J. Adams, A.I. Cooper, Angew. Chem. Int. Ed. 51, 12727–12731 (2012)
M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Chem. Soc. Rev. 38, 1330–1352 (2009)
D. Maspoch, D. Ruiz-Molina, J. Veciana, Chem. Soc. Rev. 36, 770–818 (2007)
C.L. Cahill, D.T. de Lilla, M. Frisch, CrystEngComm 9, 15–26 (2007)
A.C. Tella, S.O. Owalude, M.F. Omotoso, S.J. Olatunji, A.S. Ogunlaja, L.O. Alimi, O.K. Popoola, S.A. Bourne, J. Mol. Struct. 1157, 450–456 (2018)
X.B. Yin, Y.N. Song, Y. Wang, L.J. Zhang, Q.W. Li, Sci. China Chem. 57, 135–140 (2014)
W.J. Zhang, D.Y. Li, B. Song, J. Inorg. Organomet. Polym. 30, 3989–3998 (2020)
M.S. Deenadayalan, N. Sharma, P.K. Verma, C.M. Nagaraja, Inorg. Chem. 55, 5320–5327 (2016)
A. Halder, S. Patra, B. Viswanath, N. Munichandraiah, N. Ravishankar, Nanoscale 3, 725–730 (2011)
J. Jaramillo-García, V. Sanchez-Mendieta, I. García-Orozco, R.A. Morales-Luckie, D. Martínez-Otero, A. Téllez-López, L.D. Rosales-Vázquez, R. Escudero, F. Morales, Z. Anorg. Allg. Chem. 644, 19–22 (2018)
N. Getachew, Y. Chebule, I. Diaz, M. Sanchez-Sanchez, J. Porous Mat. 21, 769–773 (2014)
A. Nimmermark, L. Ohrstrom, J. Reedijk, Z. Kristallogr. 228, 311–317 (2013)
S.E.H. Etaiw, M.M. El-bendary, J. Inorg. Organomet. Polym. 23, 510–518 (2013)
A.C. Tella, A.C. Oladipo, V.O. Adimula, O.A. Ameen, S.A. Bourne, A.S. Ogunlaja, New J. Chem. 43, 14343–14354 (2019)
A. Phan, A.U. Czaja, F. Gandara, C.B. Knobler, O.M. Yaghi, Inorg. Chem. 50, 7388–7390 (2011)
X. Huang, S. Jiang, M. Liu, J. Phys. Chem. 109, 114–119 (2005)
V.B. Nagaveni, K.M. Mahadevan, G.R. Vijayakumar, H. Nagabhushana, S. Naveen, N.K. Lokanath, JSAMD 3, 51–58 (2018)
S.E.H. Etaiw, T.A. Fayed, D.M.A. El-Aziz, H.M. Khattab, J. Inorg. Organomet. Polym. (2021). https://doi.org/10.1007/s10904-020-01856-4
P. Horlescu, D. Sutiman, C.S. Stan, C. Mita, C. Peptu, M.E. Fortuna, C. Albu, Environ. Eng. Manag. J. 14, 389–397 (2015)
F. Moura de Oliveira, L.R. Bezerra de Araújo Nascimento, C.M. Santos-Calado, M.R. Meneghetti, M.G. Angelo da Silva, Catalysts 6(12), 215 (2016)
B. Angelov, A. Angelova, S.K. Filippov, T. Narayanan, M. Drechsler, P. Stepanek, P. Couvreur, S. Lesieur, J. Phys. Chem. Lett. 4, 1959–1964 (2013)
A. Gangula, R. Podila, M. Ramakrishna, L. Karanam, C. Janardhana, A.M. Rao, Langmuir 27, 15268–15274 (2011)
A.I. Ayad, D. Luart, A.O. Dris, E. Guénin, Nanomaterials 10, 1169 (2020)
L. Yan, L. Liu, M. Yuan, R. Guo, Colloids Surf. A Physicochem. Eng. Asp. 417, 18–25 (2013)
Y.S. Seo, E.-Y. Ahn, J. Park, T.Y. Kim, J.E. Hong, K. Kim, Y. Park, Y. Park, Nanoscale Res. Let. 12, 7 (2017)
R.K. Narayanan, S.J. Devaki, Ind. Eng. Chem. Res. 54, 1197–1203 (2015)
N. Getachew, Y. Chebule, I. Diaz, M. Sanchez-Sanchez, J. Porous Mater. 21, 769–773 (2014)
S. Bai, X. Shen, G. Zhu, M. Li, H. Xi, K. Chen, ACS Appl. Mater. Interfaces 4, 2378–2381 (2012)
W. Wu, M. Lei, S. Yang, L. Zhou, L. Liu, X. Xiao, C. Jiang, V. Roy, J. Mater. Chem. A 3, 3450–3455 (2015)
M. Tranchant, A. Serrà, C. Gunderson, E. Bertero, J. García-Amorós, E. Gómez, J. Michler, L. Philippe, Appl. Catal. A Gen. 602, 117698 (2020)
Acknowledgements
VOA is grateful to TWAS-CIIT for the 2017 TWAS-CIIT Fellowship award to carry out part of this research at the COMSATS Institute of Information Technology, Islamabad, Abbottabad Campus, Pakistan. Many thanks to Prof. M. Nawaz Tahir of the University of Sargodha, Pakistan, for helping out with the single-crystal X-ray diffraction analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tella, A.C., Owalude, S.O., Adimula, V.O. et al. Synthesis, Structure, and Properties of a Dinuclear Cu(II) Coordination Polymer Based on Quinoxaline and 3,3-Thiodipropionic Acid Ligands. J Inorg Organomet Polym 31, 3089–3100 (2021). https://doi.org/10.1007/s10904-021-01966-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01966-7