Abstract
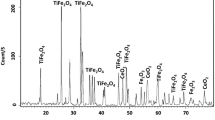
To improve the adsorption capacities of ferroferric oxide (Fe3O4) for heavy metals, cerium/ferroferric oxide composites (Ce/Fe3O4) were prepared by a chemical coprecipitation method, and the adsorption capacity of chromium (Cr) from solution by Ce/Fe3O4 was studied. According to the TEM and SEM–EDS results, Ce was successfully doped on Fe3O4, the morphology of Ce/Fe3O4 was irregular and glossy, there were no obvious pores, and distinct boundaries were apparent. According to the XRD results, Ce/Fe3O showed high crystallinity. The crystal grain size of Ce/Fe3O4 was 27.9 nm. The results showed that Ce could increase the crystallinity and crystal size of Fe3O4. The results of batch adsorption experiments, including pH dependence, coexisting ion interference, adsorption kinetics and isotherms, showed that acidic conditions are more favorable than alkaline conditions for Ce/Fe3O4 to adsorb and remove Cr(VI). However, under acidic conditions, Cr(VI) exists in the form of HCrO4−, and Cl−, NO3− and SO42− existing in the solution compete with HCrO4− for adsorption. The adsorption process is mainly physical adsorption, and it can occur spontaneously.










Similar content being viewed by others
References
J. Martino, A. Holmes, S.S. Wise, H. Xie, J.P. Wise, The role of centrosomes in chemical carcinogenesis: hexavalent chromium induces aberrant centriole and centrosome separation and centrosome amplification. Toxicol. Lett. 259, S33 (2016)
B. Zhang, Y. Wu, Y. Fan, Synthesis of novel magnetic NiFe2O4nanocomposite grafted chitosan and the adsorption mechanism of Cr(VI). J. Inorg. Organomet. Polym. Mater. 29, 290–301 (2018)
M.S. Jabir, U.M. Nayef, W.K. Abdulkadhim, Z.J. Taqi, G.M. Sulaiman, U.I. Sahib, A.M. Al-Shammari, Y.-J. Wu, M. El-Shazly, C.-C. Su, Fe3O4 nanoparticles capped with peg induce apoptosis in breast cancer amj13 cells via mitochondrial damage and reduction of Nf-Κb translocation. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01791-4
P. Srivastava, R. Abbassi, A. Kumar Yadav, V. Garaniya, N. Kumar, S.J. Khan, T. Lewis, Enhanced chromium(VI) treatment in electroactive constructed wetlands: influence of conductive material. J. Hazard. Mater. 387, 1–12 (2019)
S. Shoukat et al., Remediation of chromium(VI) and rhodamine 6G via mixed phase nickel-zinc nanocomposite: synthesis and characterization. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01776-3
P. Gao, X. Chen, F. Shen, G. Chen, Removal of chromium(VI) from wastewater by combined electrocoagulation-electroflotation without a filter. Sep. Purif. Technol. 43, 117–123 (2005)
S.K. Mondal, P. Saha, Removal of hexavalent chromium from wastewater using supported liquid membrane: synthesis of chromium-iron complex through electrochemical reaction. Water. Environ. J. 34(S1), 753–771 (2020)
A. Scarselli, A. Binazzi, D.D. Marzio, A. Marinaccio, S. Iavicoli, Hexavalent chromium compounds in the workplace: assessing the extent and magnitude of occupational exposure in Italy. J. Occup. Environ. Hyg. 9, 398–407 (2012)
R.A. Kearley, Properties of sodium chromate, sodium dichromate, and potassium dichromate, and their aqueous solutions. J. Chem. Eng. Data 9, 548–551 (1964)
J. Lou, C. Chang, Completely treating heavy metal laboratory waste liquid by an improved ferrite process. Sep. Purif. Technol. 57, 513–518 (2007)
S.A. Cavaco, S. Fernandes, M.M. Quina, L.M. Ferreira, Removal of chromium from electroplating industry effluents by ion exchange resins. J. Hazard. Mater. 144, 634–638 (2007)
W. Wang, X.J. Wang, X. Wang, L.Z. Yang, Z. Wu, S.Q. Xia, J.F. Zhao, Cr(VI) removal from aqueous solution with bamboo charcoal chemically modified by iron and cobalt with the assistance of microwave. J. Environ. Sci. China 25, 1726–1735 (2013)
A.S.K. Kumar, S. Kalidhasan, V. Rajesh, N. Rajesh, Application of cellulose-clay composite biosorbent toward the effective adsorption and removal of chromium from industrial wastewater. Ind. Eng. Chem. Res. 51, 58–69 (2011)
H.Y. Xiao, Z.H. Ai, L.Z. Zhang, Nonaqueous sol-gel synthesized hierarchical CeO2nanocrystal microspheres as novel adsorbents for wastewater treatment. J. Phys. Chem. C 113, 16625–16630 (2009)
H.X. Zhong, Y.L. Ma, X.F. Cao, X.T. Chen, Z.L. Xue, Preparation and characterization of flowerlike Y-2(OH)(5)NO3 center dot 1.5H(2)O and Y2O3 and their efficient removal of Cr(VI) from aqueous solution. J. Phys. Chem. C 113, 3461–3466 (2009)
Y.B. Yang, L.C. Zheng, T. Zhang, H.J. Yu, Y.R. Zhan, Y.F. Yang, H. Zeng, S.K. Chen, D. Peng, Adsorption behavior and mechanism of sulfonamides on phosphonic chelating cellulose under different pH effects. Bioresour. Technol. 288, 121510 (2019)
N. Amirmahani, H. Mahdizadeh, M. Malakootian, A. Pardakhty, N.O. Mahmoodi, Evaluating nanoparticles decorated on Fe3O4@SiO2-Schiff base (Fe3O4@SiO2-Aptms-Hba) in adsorption of ciprofloxacin from aqueous environments. J. Inorg. Organomet. Polym. Mater. 30, 3540–3551 (2020)
A. Bilgi, A. Cimen, Removal of chromium(VI) from polluted wastewater by chemical modification of silica gel with 4-acetyl-3-hydroxyaniline. RSC. Adv. 9(64), 37403–37414 (2019)
S. Mitra, A. Sarkar, S. Sen, Removal of chromium from industrial effluents using nanotechnology: a review. Nanotechnol. Environ. Eng. 2, 11 (2017)
Y.L. Ding, F.T. Liu, Q.H. Jiang, B. Du, H.D. Sun, 12-Hydrothermal synthesis and characterization of Fe3O4nanorods. J. Inorg. Organomet. Polym. Mater. 23, 379–384 (2013)
H. Wang, Y. Lin, Y. Li, A. Dolgormaa, H. Fang, L. Guo, J. Huang, J. Yang, A novel magnetic Cd(II) ion-imprinted polymer as a selective sorbent for the removal of cadmium ions from aqueous solution. J. Inorg. Organomet. Polym. Mater. 29, 1874–1885 (2019)
A. Baykal, M. Senel, B. Unal, E. Karaoğlu, H. Sözeri, M.S. Toprak, Acid functionalized multiwall carbon nanotube/magnetite (MWCNT)-COOH/Fe3O4 hybrid: synthesis, characterization and conductivity evaluation. J. Inorg. Organomet. Polym. Mater. 23, 726–735 (2013)
Z.H. Dastgerdi, S.S. Meshkat, S. Hosseinzadeh, M.D. Esrafili, Application of novel Fe3O4–polyanilinenanocomposites in asphaltene adsorptive removal: equilibrium, kinetic study and DFT calculations. J. Inorg. Organomet. Polym. Mater. 29, 1160–1170 (2019)
M.H. Moghim, S.M. Zebarjad, Fabrication and structural characterization of multi-walled carbon nanotube/Fe3O4nanocomposite. J. Inorg. Organomet. Polym. Mater. 25, 1260–1266 (2015)
A. Zarei, S. Saedi, F. seidi, Synthesis and application of Fe3O4@SiO2@Carboxyl-terminated pamamdendrimernanocomposite for heavy metal removal. J. Inorg. Organomet. Polym. Mater. 28, 2835–2843 (2018)
L. Dong, D. Liu, H. Fu, X. Li, L. Shan, Synthesis and photocatalytic activity of Fe3O4–WO3–CQD multifunctional system. J. Inorg. Organomet. Polym. Mater. 29, 1297–1304 (2019)
B. Li, D. Jia, Y. Zhou, Q. HU, W. Cai, In situ hybridization to chitosan/magnetite nanocomposite induced by the magnetic field. J. Magn. Magn. Mater. 306, 223–227 (2006)
S. Peng, H. Meng, Y. Ouyang, J. Chang, Nanoporous magnetic cellulose-chitosan composite microspheres: preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 53, 2106–2113 (2014)
Z. Yu, X. Zhang, Y. Huang, Magnetic chitosan–iron(III) hydrogel as a fast and reusable adsorbent for chromium(VI) removal. Ind. Eng. Chem. Res. 52, 11956–11966 (2013)
P. Yin, Y. Deng, L. Zhang, N. Li, X. Feng, J. Wang, Y. Zhang, Facile synthesis and microwave absorption investigation of activated carbon@Fe3O4 composites in the low frequency band. RSC. Adv. 8, 23048–23057 (2018)
G. Zhang, J. Qu, H. Liu, A.T. Cooper, R. Wu, CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 68, 1058–1066 (2007)
S. Pirsa, F. Asadzadeh, I. KarimiSani, Synthesis of magnetic gluten/pectin/Fe3O4nano-hydrogel and its use to reduce environmental pollutants from lakeUrmia sediments. J. Inorg. Organomet. Polym. Mater. 30, 3188–3198 (2020)
N.C. Joshi, A. Gaur, A. Singh, Synthesis, characterisations, adsorptive performances and photo-catalytic activity of Fe3O4-SiO2 based nanosorbent (Fe3O4-SiO2Bn). J. Inorg. Organomet. Polym. Mater. 30, 4416–4425 (2020)
C. Doerenkamp, E. Carvajal, C.J. Magon, W.J.G.J. Faria, J.P. Donoso, Y. GalvãoGobato, A.S.S. de Camargo, H. Eckert, Composition–structure–property correlations in rare-earth-doped heavy metal oxyfluoride glasses. J. Phys. Chem. C 123, 22478–22490 (2019)
D. Zhai, Y. Shui, K. Feng, Y. Zhang, Effects of rare-earth oxides on the microstructure and properties of Fe-based friction materials synthesized by in situ carbothermic reaction from vanadium-bearing titanomagnetite concentrates. RSC. Adv. 9, 20687–20697 (2019)
R. Srivastava, Eco-friendly and morphologically-controlled synthesis of porous CeO2 microstructure and its application in water purification. J. Colloid. Interface. Sci. 348, 600–607 (2010)
P. Liang, Y. Zhang, D.F. Wang, Y. Xu, L. Luo, Preparation of mixed rare earths modified chitosan for fluoride adsorption. J. Rare. Earth. 31, 817–822 (2013)
H. Paudyal, B. Pangeni, K. Inoue, H. Kawakita, K. Ohto, K.N. Ghimire, H. Harada, S. Alam, Adsorptive removal of trace concentration of fluoride ion from water by using dried orange juice residue. Chem. Eng. J. 223, 844–853 (2013)
V. Sivasankar, S. Murugesh, S. Rajkumar, A. Darchen, Cerium dispersed in carbon (Cedc) and its adsorption behavior: a first example of tailored adsorbent for fluoride removal from drinking water. Chem. Eng. J. 214, 45–54 (2013)
S.A. Wasay, M.J. Haran, S. Tokunaga, Adsorption of fluoride, phosphate, and arsenate ions on lanthanum-impregnated silica gel. Water. Environ. Res. 68, 295–300 (1996)
N.M. Zúñiga-Muro, A. Bonilla-Petriciolet, D.I. Mendoza-Castillo, H.E. Reynel-Ávila, J.C. Tapia-Picazo, Fluoride adsorption properties of cerium-containing bone char. J. Fluorine. Chem. 197, 63–73 (2017)
X.T. Lin, S.J. Li, H. He, Z. Wu, J.L. Wu, L.M. Chen, D.Q. Ye, M.L. Fu, Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B 223, 91–102 (2018)
Y. Seida, Y. Izumi, Synthesis of clay-cerium hydroxide conjugates for the adsorption of arsenic. Adsorpt. Sci. Technol. 23, 607–618 (2005)
H. Kavas, M. Gunay, A. Baykal, M.S. Toprak, H. Sozeri, B. Aktas, Negative permittivity of polyaniline-Fe3O4nanocomposite. J. Inorg. Organomet. Polym. Mater. 23, 306–314 (2013)
P. Zhang, S.D. Ouyang, P. Li, Z.Y. Sun, N.S. Ding, Y. Huang, Ultrahigh removal performance of lead from wastewater by tricalcium aluminate via precipitation combining flocculation with amorphous aluminum. J. Clean. Prod. 246, 118728 (2020)
J. Hu, G. Chen, I.M. Lo, Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water. Res. 39, 4528–4536 (2005)
Z. Razmara, E. Sanchooli, Hydrothermal synthesis of Co(II) complex, a precursor for the synthesis of octahedral Co3O4 nanoparticles : an active catalyst for the removal of Cr(VI). J. Inorg. Organomet. Polym. Mater. 29, 2090–2102 (2019)
T. Shi, Z. Wang, Y. Liu, S. Jia, D. Changming, Removal of hexavalent chromium from aqueous solutions by D301, D314 and D354 anion-exchange resins. J. Hazard. Mater. 161, 900–906 (2009)
K.S. Tong, M.J. Kassim, A. Azraa, Adsorption of copper ion from its aqueous solution by a novel biosorbentuncariagambir: equilibrium, kinetics, and thermodynamic studies. Chem. Eng. J. 170, 145–153 (2011)
G. Wójcik, V. Neagu, I. Bunia, Sorption studies of chromium(VI) onto new ion exchanger with tertiary amine, quaternary ammonium and ketone groups. J. Hazard. Mater. 190, 544–552 (2011)
M. Luo, H. Lin, B. Li, Y. Dong, Y. He, L. Wang, A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour. Technol. 259, 312–318 (2018)
P.K. Bajpai, C. Goel, H. Bhunia, Mesoporous carbon adsorbents from melamine-formaldehyde resin using nanocasting technique for Co2 adsorption. J. Environ. Sci. 32, 238–248 (2015)
S. Yu, X. Wang, Z. Chen, J. Wang, X. Wang, Layered double hydroxide intercalated with aromatic acid anions for the efficient capture of aniline from aqueous solution. J. Hazard. Mater. 321, 111–120 (2016)
S.J. Yu, J. Wang, S. Song, K.Y. Sun, J. Li, X.X. Wang, Z.S. Chen, X.K. Wang, One-pot synthesis of graphene oxide and Ni-Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater. Sci. China. Chem. 60, 415–422 (2017)
H. Pang, Z. Diao, X. Wang, Y. Ma, S. Yu, H. Zhu, Z. Chen, B. Hu, J. Chen, X. Wang, Adsorptive and reductive removal of U(VI) by dictyophoraindusiate-derived biochar supported sulfide nZVI from wastewater. Chem. Eng. J. 366, 368–377 (2019)
A. Kilislioglu, B. Bilgin, Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl. Radiat. Isot. 58, 155–160 (2003)
Acknowledgement
This paper is sponsored by the Chinese Research Academy of Environmental Sciences support program (Grant Number 2012BAJ21B08-05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, B., Wu, L., Ou, M. et al. Sorption Studies of Chromium(VI) onto Cerium/Ferroferric Oxide Composites. J Inorg Organomet Polym 31, 2627–2637 (2021). https://doi.org/10.1007/s10904-021-01944-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01944-z