Abstract
In this study novel biopolymer and water soluble O-carboxymethyl chitosan Schiff base derivatives have been designed for potential use in biological applications. Chemically modified O-carboxymethyl chitosan Schiff base and their metal complexes were prepared for potential application in antibacterial, antifungal, anti-inflammatory, total antioxidant, antidiabetic avenues. Chitosan possess water insolubility has been eliminated by carboxymethylation. The thermal decompositions of metal complexes results that the chitosan polymer had more thermal stability than Schiff base ligand and their Co (II), Ni (II) and Zn (II) metal complexes. The antibacterial results showed that the Ni (II) metal complexes had more efficacies against the bacteria and fungi. The anti-inflammatory studies of compounds reveals that the nickel (II) complex was denatured about 91.88 ± 3.1% of bovine serum albumin (BSA). The biological studies proved that the synthesized compounds possess better antibacterial, antifungal, anti-inflammatory, antidiabetic and antioxidant in nature.
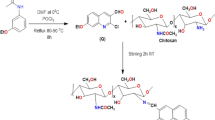
Graphic Abstract









Similar content being viewed by others
References
K. Kurita, Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. 8(3), 203–226 (2006). https://doi.org/10.1007/s10126-005-0097-5
S.S. Silva, J.F. Mano, R.L. Reis, Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 19(5), 1208–1220 (2017). https://doi.org/10.1039/c6gc02827f
K. Mohan, S. Ravichandran, T. Muralisankar, V. Uthayakumar, R. Chandirasekar, C. Rajeevgandhi, P. Seedevi, Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int. J. Biol. Macromol. (2018). https://doi.org/10.1016/j.ijbiomac.2018.12.241
H.S. Adhikari, P.N. Yadav, Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomater. 2018, 1–29 (2018). https://doi.org/10.1155/2018/2952085
A. Tolaimate, J. Desbrières, M. Rhazi, A. Alagui, M. Vincendon, P. Vottero, On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer 41(7), 2463–2469 (2000). https://doi.org/10.1016/s0032-3861(99)00400-0
K. Muraleedharan, C.H. Viswalekshmi, K. Sarada, Synthesis, characterization and thermal dehydration and degradation kinetics of chitosan Schiff bases of o-, m- and p-nitrobenzaldehyde. Polym. Bull. 74(1), 39–54 (2016). https://doi.org/10.1007/s00289-016-1696-1
A.L. Bukzem, R. Signini, D.M. dos Santos, L.M. Lião, D.P.R. Ascheri, Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 85, 615–624 (2016). https://doi.org/10.1016/j.ijbiomac.2016.01.017
F.R. De Abreu, S.P. Campana-Filho, Characteristics and properties of carboxymethylchitosan. Carbohyd. Polym. 75(2), 214–221 (2009). https://doi.org/10.1016/j.carbpol.2008.06.009
D.N. Dhar, C.L. Taploo, Schiff bases and their applications. J. Sci. Ind. Res. 41, 501–506 (1982)
P. Przybylski, A. Huczynski, K. Pyta, B. Brzezinski, F. Bartl, Biological properties of schiff bases and azo derivatives of phenols. Curr. Org. Chem. 13(2), 124–148 (2009). https://doi.org/10.2174/138527209787193774
R. Antony, T. Arun, S.T.D. Manickam, A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. (2019). https://doi.org/10.1016/j.ijbiomac.2019.02.047
K. Czarnek, S. Terpiłowska, A.K. Siwicki, Review paper: Selected aspects of the action of cobalt ions in the human body. Central Eur. J. Immunol. 2, 236–242 (2015). https://doi.org/10.5114/ceji.2015.52837
M. Manimohan, S. Pugalmani, M.A. Sithique, Biologically active novels N, N, O donor tridentate water soluble hydrazide based O-carboxymethyl chitosan Schiff base Cu (II) metal complexes: synthesis and characterisation. Int. J. Biol. Macromol. 136, 738–754 (2019). https://doi.org/10.1016/j.ijbiomac.2019.06.115
M. Murugaiyan, S.P. Mani, M.A. Sithique, Zinc (ii) centered biologically active novel N, N, O donor tridentate water-soluble hydrazide-based O-carboxymethyl chitosan Schiff base metal complexes: synthesis and characterisation. New J. Chem. 43, 9540–9554 (2019). https://doi.org/10.1039/c9nj00670b
M. Manimohan, S. Pugalmani, M.A. Sithique, Biologically active water soluble novel biopolymer/hydrazide based O-carboxymethyl chitosan Schiff bases: synthesis and characterisation. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01487-9
T. Baran, A. Menteş, Polymeric material prepared from Schiff base based on O-carboxymethyl chitosan and its Cu(II) and Pd(II) complexes. J. Mol. Struct. 1115, 220–227 (2016). https://doi.org/10.1016/j.molstruc.2016.03.015
W. Liu, Y. Qin, S. Liu, R. Xing, H. Yu, X. Chen et al., Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 114, 942–949 (2018). https://doi.org/10.1016/j.ijbiomac.2018.03.179
R. Antony, S. Theodore David Manickam, K. Saravanan, K. Karuppasamy, S. Balakumar, Synthesis, spectroscopic and catalytic studies of Cu(II), Co(II) and Ni(II) complexes immobilized on Schiff base modified chitosan. J. Mol. Struct. 1050, 53–60 (2013). https://doi.org/10.1016/j.molstruc.2013.07.006
D. Egli, R. Baumann, S. Küng, A. Berger, L. Baron, M. Herwegh, Structural characteristics, bulk porosity and evolution of an exhumed long-lived hydrothermal system. Tectonophysics (2018). https://doi.org/10.1016/j.tecto.2018.10.008
T. Baran, A. Menteş, Construction of new biopolymer (chitosan)-based pincer-type Pd(II) complex and its catalytic application in Suzuki cross coupling reactions. J. Mol. Struct. 1134, 591–598 (2017). https://doi.org/10.1016/j.molstruc.2017.01.005
K.R. Krishnapriya, M. Kandaswamy, A new chitosan biopolymer derivative as metal-complexing agent: synthesis, characterization, and metal(II) ion adsorption studies. Carbohyd. Res. 345(14), 2013–2022 (2010). https://doi.org/10.1016/j.carres.2010.06.005
S. Aslkhademi, N. Noshiranzadeh, M.S. Sadjadi, K. Mehrani, N. Farhadyar, Synthesis, crystal structure and investigation of the catalytic and spectroscopic properties of a Zn(II) complex with Coumarin-hydrazone ligand. Polyhedron (2018). https://doi.org/10.1016/j.poly.2018.12.023
Q. Song, Z. Zhang, J. Gao, C. Ding, Synthesis and property studies of N-carboxymethyl chitosan. J. Appl. Polym. Sci. 119(6), 3282–3285 (2010). https://doi.org/10.1002/app.32925
S. Yadav, I. Yousuf, M. Usman, M. Ahmad, F. Arjmand, S. Tabassum, Synthesis and spectroscopic characterization of diorganotin (iv) complexes of N′-(4-hydroxypent-3-en-2-ylidene) isonicotinohydrazide: chemotherapeutic potential validation by in vitro interaction studies with DNA/HSA, DFT, molecular docking and cytotoxic activity. RSC Adv. 5(63), 50673–50690 (2015). https://doi.org/10.1039/c5ra06953j
E.L. De Araújo, H.F.G. Barbosa, E.R. Dockal, ÉT.G. Cavalheiro, Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int. J. Biol. Macromol. 95, 168–176 (2017). https://doi.org/10.1016/j.ijbiomac.2016.10.109
A.A. Alhwaige, H. Ishida, S. Qutubuddin, Poly (benzoxazine-f-chitosan) films: the role of aldehyde neighboring groups on chemical interaction of benzoxazine precursors with chitosan. Carbohydr. Polym. (2019). https://doi.org/10.1016/j.carbpol.2019.01.016
A.B. Muley, M.R. Ladole, P. Suprasanna, S.G. Dalvi, Intensification in biological properties of chitosan after γ-irradiation. Int. J. Biol. Macromol. (2019). https://doi.org/10.1016/j.ijbiomac.2019.03.072
O.A.M. Ali, S.M. El-Medani, D.A. Ahmed, D.A. Nassar, Synthesis, characterization, fluorescence and catalytic activity of some new complexes of unsymmetrical Schiff base of 2-pyridinecarboxaldehyde with 2, 6-diaminopyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 144, 99–106 (2015). https://doi.org/10.1016/j.saa.2015.02.078
L. Wei, Y. Mi, J. Zhang, Q. Li, F. Dong, Z. Guo, Evaluation of quaternary ammonium chitosan derivatives differing in the length of alkyl side-chain: synthesis and antifungal activity. Int. J. Biol. Macromol. 129, 1127–1132 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.099
H. Barbosa, M. Attjioui, A. Ferreira, E. Dockal, N. El Gueddari, B. Moerschbacher, É Cavalheiro, Synthesis, characterization and biological activities of biopolymeric schiff bases prepared with chitosan and salicylaldehydes and their Pd(II) and Pt(II) complexes. Molecules 22(11), 1987 (2017). https://doi.org/10.3390/molecules22111987
R. Sribalan, M. Kirubavathi, G. Banuppriya, V. Padmini, Synthesis and biological evaluation of new symmetric curcumin derivatives. Bioorg. Med. Chem. Lett. 25(19), 4282–4286 (2015). https://doi.org/10.1016/j.bmcl.2015.07.088
T.S. Saranya, V.K. Rajan, R. Biswas, R. Jayakumar, S. Sathianarayanan, Synthesis, characterisation and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 110, 227–233 (2018). https://doi.org/10.1016/j.ijbiomac.2017.12.044
J. Zhang, W. Tan, G. Wang, X. Yin, Q. Li, F. Dong, Z. Guo, Synthesis, characterization, and the antioxidant activity of N, N, N -trimethyl chitosan salts. Int. J. Biol. Macromol. 118, 9–14 (2018)
T.M. Chaouche, F. Haddouchi, R. Ksouri, F. Atik-Bekkara, Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 77(6), 302–307 (2014)
W. Zhu, Z. Zhang, Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int. J. Biol. Macromol. 70, 150–155 (2014). https://doi.org/10.1016/j.ijbiomac.2014.06.047
Y. Liu, S. Zeng, Y. Liu, W. Wu, Y. Shen, L. Zhang, C. Wang, Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 114, 632–639 (2018). https://doi.org/10.1016/j.ijbiomac.2018.03.161
Acknowledgements
The authors are thankful to the PG and Research Department of Chemistry, Islamiah College (Autonomous), Vaniyambadi, Tirupattur (Dt), Tamil Nadu, India for providing the laboratory and the Instrumentation facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manimohan, M., Pugalmani, S. & Sithique, M.A. Synthesis, Spectral Characterisation and Biological Activities of Novel Biomaterial/N, N, O Donor Tridentate Co (II), Ni (II) and Zn (II) Complexes of Hydrazide Based Biopolymer Schiff Base Ligand. J Inorg Organomet Polym 30, 4481–4495 (2020). https://doi.org/10.1007/s10904-020-01578-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01578-7