Abstract
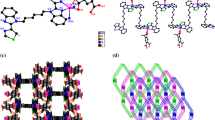
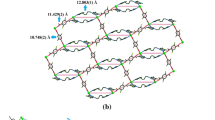
Five coordination polymers (CPs) {[Zn4(FDA)4(H2O)12]}n (1) {[M(FDA)(azopy)·DMF]}n (2) [M = Zn (2a), Co (2b) and Cd (2c)] and {[Zn(FDA)(azopy)]·CH3CN·0.5DMF}n (3) have been harvested from furan-2,5-dicarboxylic acid (H2FDA) and 4,4′-azobispyridine (azopy), and structurally characterized by infrared spectra, elemental analysis, thermogravimentric analysis, powder X-ray diffraction and single-crystal X-ray diffraction. CP 1 shows an interesting one-dimension chain structure. CP 2a and CP 2b are isostructural and demonstrate two-dimensional 4-connected sql topological structures where FDA2− connected the nearest Zn(II) ions to generate a one-dimension chain and the chains could be further linked by azopy. CP 2c significantly differs from CP 2a and CP 2b in coordination number as well as in the coordination mode of carboxylate groups. However CP 2c can generate the same two-dimensional sql structure as well. While CP 3 is an interesting pillared-layer three-dimensional two-fold interpenetrating framework with a pcu alpha-Po primitive cubic topology. The luminescence properties of CP 1, CP 2a, CP 2c and CP 3 are investigated.






Similar content being viewed by others
References
S.R. Batten, S.M. Neville, D.R. Turner, Coordination Polymers Design, Analysis and Application (Royal Society of Chemistry, Cambridge, 2009), www.rsc.org
M. O’Keeffe, O.M. Yaghi, Chem. Rev. 112, 675–702 (2012)
Z.J. Lin, M.C. Hong, R. Cao, Chem. Soc. Rev. 43, 5867–5895 (2014)
S. Kitagawa, Chem. Soc. Rev. 43, 5415–5418 (2014)
F. Luo, C.S. Yan, L.L. Dang, R. Krishna, W. Zhou, H. Wu, X.L. Dong, Y. Han, T.L. Hu, M. O’Keeffe, L.L. Wang, M.B. Luo, R.B. Lin, B.L. Chen, J. Am. Chem. Soc. 138, 5678–5684 (2016)
J. Duan, M. Higuchi, R. Krishna, T. Kiyonaga, Y. Tsutsumi, Y. Sato, Y. Kubota, M. Takata, S. Kitagawa, Chem. Sci. 5, 660–666 (2014)
F. Moro, D. Kaminski, F. Tuna, G.F.S. Whitehead, G.A. Timco, D. Collison, R.E.P. Winpenny, A. Ardavan, E.J.L. Mclnnes, Chem. Commun. 50, 91–93 (2014)
S.W. Zhang, P. Cheng, ChemPlusChem 81, 811–816 (2016)
J.Y. Zou, W. Shi, N. Xu, L.L. Li, J.K. Tang, H.L. Gao, J.Z. Cui, P. Cheng, Chem. Commun. 49, 8226–8228 (2013)
S.W. Zhang, H. Li, E.Y. Duan, Z.S. Han, L.L. Li, J.K. Tang, W. Shi, P. Cheng, Inorg. Chem. 55, 1202–1207 (2016)
Z.L. Wu, J. Dong, W.Y. Ni, B.W. Zhang, J.Z. Cui, B. Zhao, Inorg. Chem. 54, 5266–5272 (2015)
Y. Xie, S.G. Ning, Y. Zhang, Z.L. Tang, S.W. Zhang, R.R. Tang, Dyes Pigm. 150, 36–43 (2018)
Y. Wang, P. Xu, Q. Xie, Q.Q. Ma, Y.H. Meng, Z.W. Wang, S.W. Zhang, X.H. Zhao, J. Chen, Z.L. Wang, Chem. Eur. J. 22, 10459–10474 (2016)
G. Maurin, C. Serre, A. Cooper, G. Férey, Chem. Soc. Rev. 46, 3104–3107 (2017)
Z. Ju, S. Yan, D. Yuan, Chem. Mater. 28, 2000–2010 (2016)
X.X. Zhao, S.W. Zhang, J.Q. Yan, L.D. Li, G.J. Wu, W. Shi, G.M. Yang, N.J. Guan, P. Cheng, Inorg. Chem. 57, 5030–5037 (2018)
J.Y. Zou, W. Shi, H.L. Gao, J.Z. Cui, P. Cheng, Inorg. Chem. Front. 1, 242–248 (2014)
W.P. Chen, P.Q. Liao, Y. Yu, Z. Zheng, X.M. Chen, Y.Z. Zheng, Angew. Chem. Int. Ed. 55, 9375–9379 (2016)
S.W. Zhang, W. Shi, P. Cheng, Coord. Chem. Rev. 352, 108–150 (2017)
S. Horike, S. Kitagawa, Nat. Mater. 16, 1054–1055 (2017)
Z. Hasan, S.H. Jhung, J. Hazard. Mater. 283, 329–339 (2015)
Q.G. Zhai, X.H. Bu, C.Y. Mao, X. Zhao, L. Daemen, Y.Q. Chen, A.J. Ramirez-Cuesta, C.Y. Mao, Nat. Commun. 7, 1–9 (2016)
S.G. Ning, H.J. Chen, S.W. Zhang, P. Cheng, Polyhedron 155, 457–463 (2018)
Y.L. Wu, G.P. Yang, Y.Q. Zhao, W.P. Wu, B. Liu, Y.Y. Wang, Dalton Trans. 44, 3271–3277 (2015)
F.L. Hu, F.L. Jiang, J. Zheng, M.Y. Wu, J.D. Pang, M.C. Hong, Inorg. Chem. 54, 6081–6083 (2015)
Y. Wei, R. Sa, Q. Li, K. Wu, Dalton Trans. 44, 3067–3074 (2015)
Z.L. Tang, H.J. Chen, Y. Zhang, B.S. Zheng, S.W. Zhang, P. Cheng, Cryst. Growth Des. 19, 1172–1182 (2019)
Q.F. Sun, S. Sato, M. Fujita, Angew. Chem. Int. Ed. 53, 13510–13513 (2014)
L. Li, J.Y. Zou, S.Y. You, H.M. Cui, G.P. Zeng, J.Z. Cui, Dalton Trans. 46, 16432–16438 (2017)
H. Wang, J. Xu, D.S. Zhang, Q. Chen, R.M. Wen, Z. Cheng, X.H. Bu, Angew. Chem. Int. Ed. 54, 1–6 (2015)
K. Liu, H.H. Li, X.J. Zhang, W. Shi, P. Cheng, Inorg. Chem. 54, 10231–10244 (2015)
B. Bhattacharya, R. Dey, P. Pachfule, R. Banerjee, D. Ghoshal, Cryst. Growth Des. 13, 731–739 (2012)
J.Y. Zou, H.L. Gao, W. Shi, J.Z. Cui, P. Cheng, CrystEngComm 15, 2682–2687 (2013)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Crystallogr. 42, 339–341 (2009)
G.M. Sheldrick, Acta Crystallogr. A 71, 3–8 (2015)
G.M. Sheldrick, Acta Crystallogr. C 71, 3–8 (2015)
V.A. Blatov, A.P. Shevchenko, D.M. Proserpio, Cryst. Growth Des. 14, 3576–3586 (2014). http://topospro.com
M. O’Keeffe, O.M. Yaghi, S. Ramsden, Reticular Chemistry Structure Resource (2007). Database available at http://rcsr.anu.edu.au/
J.A. Foster, S. Henke, A. Schneemann, R.A. Fischer, A.K. Cheetham, Chem. Commun. 52, 10474–10477 (2016)
L. Wei, Q. Wei, Z.E. Lin, Q. Meng, H. He, B.F. Yang, G.Y. Yang, Angew. Chem. Int. Ed. 53, 7188–7191 (2014)
X.L. Zhao, W.Y. Sun, CrystEngComm 16, 3247–3258 (2014)
A.L. Spek, PLATON. J. Appl. Crystallogr. 36, 7–13 (2003)
S. Parshamoni, J. Telangae, S. Konar, Dalton Trans. 44, 20926–20935 (2015)
W.H. Fang, L. Zhang, J. Zhang, Chem. Commun. 53, 3949–3951 (2017)
B.K. Tripuramallu, S. Goswami, I. Goldberg, Cryst. Growth Des. 18, 230–241 (2017)
J.Y. Zou, L. Li, S.Y. You, H.M. Cui, Y.W. Liu, K.H. Chen, Y.H. Chen, J.Z. Cui, S.W. Zhang, Dyes Pigment. 159, 429–438 (2018)
R.P. Ye, X. Zhang, J.Q. Zhai, Y.Y. Zhang, Y.G. Yao, J. Zhang, CrystEngComm 17, 9155–9166 (2015)
J.A. Hua, Y. Zhao, Y.S. Kang, Y. Lu, W.Y. Sun, Dalton Trans. 44, 11524–11532 (2015)
Z.Q. Shi, Z.J. Guo, H.G. Zheng, Chem. Commun. 51, 8300–8303 (2015)
J.Y. Zou, L. Li, S.Y. You, K.H. Chen, X.N. Dong, Y.H. Chen, J.Z. Cui, Cryst. Growth Des. 18, 3997–4003 (2018)
Y. Hu, M. Ding, X.Q. Liu, L.B. Sun, H.L. Jiang, Chem. Commun. 52, 5734–5737 (2016)
Acknowledgements
Financial supports from the National Natural Science Foundation of China (Nos. 21561014 and 21562023), the Key Research Project of Jiangxi Province (No. 20171BBF60074) and the Key Research Project of Jiangxi Academy of Sciences (No. 2018-YZD2-11) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A: Supplementary Data
Appendix A: Supplementary Data
CCDC 1843748 for 1, 1843749 for 2a, 1843752 for 2b, 1843750 for 2c, and 1843754 for 3 contain the supplementary crystallographic data of this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
You, SY., Li, L., Zou, JY. et al. Assembly of Five Coordination Polymers Based on Furan-2,5-dicarboxylic acid and 4,4′-Azobispyridine: Synthesis, Structures and Luminescence Properties. J Inorg Organomet Polym 30, 410–416 (2020). https://doi.org/10.1007/s10904-019-01199-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01199-9