Abstract
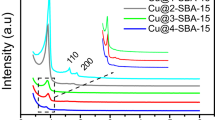
Copper nanoparticles dispersed on amine functionalised mesoporous silica SBA-15 (Cu/NH2-SBA-15) were synthesised by a simple sol–gel and impregnation method using sodium borohydride (NaBH4) as a reducing agent. The morphology, mesostructure and functionality of the ordered mesoporous Cu/NH2-SBA-15 were evaluated by powder X-ray diffraction (PXRD), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), Nitrogen adsorption–desorption isotherms (BET) and Fourier transform infrared spectroscopy (FTIR). The results obtained revealed, that the amine functionalised SBA-15 holds hexagonal lamelliform with surface area and pore size of 250 m2/g, 2.2 nm respectively. Moreover, these short vertical channels have a substantial role in the uniform dispersion of copper nanoparticles within the meso-channels of amine functionalised SBA-15. Cu nanoparticles in the size range of 4–7 nm were dispersed on the NH2-SBA-15 support. To confirm the potential catalytic activity, Cu/NH2-SBA-15 was tested in Mannich reaction. The catalyst showed an excellent catalytic activity for the yield (90%) of β-amino carbonyl compounds that serve as a building block for the synthesis of lactams, peptides, amino alcohols and precursor for various amino acids. Further, the activity of the catalyst was also tested for the reduction of dyes. The structural influence over the reduction pathways was studied on triphenyl methane dyes.
Graphical Abstract









Similar content being viewed by others
References
C. Mannich, W. Krösche, Ueber ein Kondensationsprodukt aus Formaldehyd, Ammoniak und Antipyrin. Arch. Pharm. Pharm. Med. Chem. 250, 647–667 (1912). https://doi.org/10.1002/ardp.19122500151
M. Tramontini, L. Angiolini, Mannich bases-chemistry and uses, vol. 5 (CRC Press, Boca Raton, 1994)
M. Arend, B. Westermann, N. Risch, Modern variants of the Mannich reaction. Angew. Chem. Int. Ed. 37(8), 1044–1070 (1998). https://doi.org/10.1002/(sici)1521-3773(19980504)37:8%3c1044:aid-anie1044%3e3.0.co;2-e
W.N. Speckamp, M.J. Moolenaar, New developments in the chemistry of N-acyliminium ions and related intermediates. Tetrahedron 56(24), 3817–3856 (2000). https://doi.org/10.1016/S0040-4020(00)00159-9
B.M. Trost, L.R. Terrell, A direct catalytic asymmetric Mannich-type reaction to syn-amino alcohols. J. Am. Chem. Soc. 125(2), 338–339 (2003). https://doi.org/10.1021/ja028782e
S. Matsunaga, N. Kumagai, S. Harada, M. Shibasaki, Anti-Selective direct catalytic asymmetric Mannich-type reaction of hydroxyketone providing β-amino alcohols. J. Am. Chem. Soc. 125(16), 4712–4713 (2003). https://doi.org/10.1021/ja034787f
T. Akiyama, K. Matsuda, K. Fuchibe, HCl-catalyzed stereoselective Mannich reaction in H2O-SDS system. Synlett (2005). https://doi.org/10.1055/s-2004-836062
T. Akiyama, J. Takaya, H. Kagoshima, One-pot Mannich-type reaction in water: HBF4 catalyzed condensation of aldehydes, amines, and silyl enolates for the synthesis of β-amino carbonyl compounds. Synlett 15(09), 1426–1428 (1999)
M. Periasamy, S. Suresh, S.S. Ganesan, Stereoselective synthesis of syn-β-amino esters using the TiCl 4/R 3 N reagent system. Tetrahedron Lett. 46(33), 5521–5524 (2005). https://doi.org/10.1016/j.tetlet.2005.06.048
D. Prukała, New compounds via Mannich reaction of cytosine, paraformaldehyde and cyclic secondary amines. Tetrahedron Lett. 47(51), 9045–9047 (2006). https://doi.org/10.1016/j.tetlet.2006.10.117
E. Takahashi, H. Fujisawa, T. Mukaiyama, Lithium acetate-catalyzed Mannich-type reaction between trimethylsilyl enolates and aldimines in a water-containing DMF. Chem. Lett. 33(7), 936–937 (2004). https://doi.org/10.1246/cl.2004.936
M. Kidwai, D. Bhatnagar, N.K. Mishra, V. Bansal, CAN catalyzed synthesis of β-amino carbonyl compounds via Mannich reaction in PEG. Catal. Commun. 9(15), 2547–2549 (2008). https://doi.org/10.1016/j.catcom.2008.07.010
R.K. Sharma, D. Rawat, G. Gaba, Inorganic–organic hybrid silica based tin (II) catalyst: synthesis, characterization and application in one-pot three-component Mannich reaction. Catal. Commun. 19, 31–36 (2012). https://doi.org/10.1016/j.catcom.2011.12.006
M. Kidwai, N.K. Mishra, V. Bansal, A. Kumar, S. Mozumdar, Novel one-pot Cu-nanoparticles-catalyzed Mannich reaction. Tetrahedron Lett. 50(12), 1355–1358 (2009). https://doi.org/10.1016/j.tetlet.2009.01.031
B. White, M. Yin, A. Hall, D. Le, S. Stolbov, T. Rahman, S. O’Brien, Complete CO oxidation over Cu2O nanoparticles supported on silica gel. Nano Lett. 6(9), 2095–2098 (2006). https://doi.org/10.1021/nl061457v
H. Berndt, A. Martin, A. Brückner, E. Schreier, D. Müller, H. Kosslick, G.U. Wolf, B.J. Lücke, Structure and catalytic properties of VOx/MCM materials for the partial oxidation of methane to formaldehyde. J. Catal. 191(2), 384–400 (2000). https://doi.org/10.1006/jcat.1999.2786
V. Fornés, C. Lopez, H.H. Lopez, A. Martınez, Catalytic performance of mesoporous VOx/SBA-15 catalysts for the partial oxidation of methane to formaldehyde. Appl. Catal. A 249(2), 345–354 (2003). https://doi.org/10.1016/S0926-860X(03)00226-6
M. Baltes, K. Cassiers, P. Van Der Voort, B.M. Weckhuysen, R.A. Schoonheydt, E.F. Vansant, MCM-48-supported vanadium oxide catalysts, prepared by the molecular designed dispersion of VO (acac) 2: a detailed study of the highly reactive MCM-48 surface and the structure and activity of the deposited VOx. J. Catal. 197(1), 160–171 (2001). https://doi.org/10.1006/jcat.2000.3066
G. Du, S. Lim, M. Pinault, C. Wang, F. Fang, L. Pfefferle, G.L. Haller, Synthesis, characterization, and catalytic performance of highly dispersed vanadium grafted SBA-15 catalyst. J. Catal. 253(1), 74–90 (2008). https://doi.org/10.1016/j.jcat.2007.10.019
F. Kleitz, F. Berube, R. Guillet-Nicolas, C.M. Yang, M. Thommes, Probing adsorption, pore condensation, and hysteresis behavior of pure fluids in three-dimensional cubic mesoporous KIT-6 silica. J. Phys. Chem. C 114(20), 9344–9355 (2010). https://doi.org/10.1021/jp909836v
D. Zhao, J. Sun, Q. Li, G.D. Stucky, Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater. 12(2), 275–279 (2000). https://doi.org/10.1021/cm9911363
C.H. Tu, A.Q. Wang, M.Y. Zheng, X.D. Wang, T. Zhang, Factors influencing the catalytic activity of SBA-15-supported copper nanoparticles in CO oxidation. Appl. Catal. A 297(1), 40–47 (2006). https://doi.org/10.1016/j.apcata.2005.08.035
K. Yoshida, C. Gonzalez-Arellano, R. Luque, P.L. Gai, Efficient hydrogenation of carbonyl compounds using low-loaded supported copper nanoparticles under microwave irradiation. Appl. Catal. A 379(1–2), 38–44 (2010). https://doi.org/10.1016/j.apcata.2010.02.028
C.M. Chanquía, K. Sapag, E. Rodríguez-Castellón, E.R. Herrero, G.A. Eimer, Nature and location of copper nanospecies in mesoporous molecular sieves. J. Phys. Chem. C 114(3), 1481–1490 (2010). https://doi.org/10.1021/jp9094529
B.K. Ghosh, S. Hazra, B. Naik, N.N. Ghosh, Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 269, 371–378 (2015). https://doi.org/10.1016/j.powtec.2014.09.027
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279(5350), 548–552 (1998). https://doi.org/10.1126/science.279.5350.548
S. Anbu Anjugam Vandarkuzhali, S. Karthikeyan, B. Viswanathan, M.P. Pachamuthu, Arachis hypogaea derived activated carbon/Pt catalyst: reduction of organic dyes. Surf. Interfaces 13, 101–111 (2018). https://doi.org/10.1016/j.surfin.2018.07.005
N.A. Brunelli, K. Venkatasubbaiah, C.W. Jones, Cooperative catalysis with acid–base bifunctional mesoporous silica: impact of grafting and co-condensation synthesis methods on material structure and catalytic properties. Chem. Mater. 24(13), 2433–2442 (2012). https://doi.org/10.1021/cm300753z
M.P. Pachamuthu, S. Karthikeyan, R. Maheswari, A.F. Lee, A. Ramanathan, Fenton-like degradation of Bisphenol A catalyzed by mesoporous Cu/TUD-1. Appl. Surf. Sci. 393, 67–73 (2017). https://doi.org/10.1016/j.apsusc.2016.09.162
L. Chen, J. Hu, Z. Qi, Y. Fang, R. Richards, Gold nanoparticles intercalated into the walls of mesoporous silica as a versatile redox catalyst. Ind. Eng. Chem. Res. 50(24), 13642–13649 (2011). https://doi.org/10.1021/ie200606t
R. Al-Oweini, H. El-Rossy, Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors. J. Mol. Struct. 919, 140–145 (2009). https://doi.org/10.1016/j.molstruc.2008.08.025
B.L. Newalkar, S. Komarneni, Control over microporosity of ordered microporous–mesoporous silica SBA-15 framework under microwave-hydrothermal conditions: effect of salt addition. Chem. Mater. 13(12), 4573–4579 (2001). https://doi.org/10.1021/cm0103038
Y. Zhu, H. Li, Q. Zheng, J. Xu, X. Li, Amine-functionalized SBA-15 with uniform morphology and well-defined mesostructure for highly sensitive chemosensors to detect formaldehyde vapor. Langmuir 28(20), 7843–7850 (2012). https://doi.org/10.1021/la300560j
Y.F. Shi, Y. Meng, D.H. Chen, S.J. Cheng, P. Chen, H.F. Yang, Y. Wan, D.Y. Zhao, Highly ordered mesoporous silicon carbide ceramics with large surface areas and high stability. J. Mater. Chem. 16, 1511–1519 (2006). https://doi.org/10.1002/adfm.200500643
M.P. Pachamuthu, K. Shanthi, R. Luque, A. Ramanathan, SnTUD-1: a solid acid catalyst for three component coupling reactions at room temperature. RSC Green Chem. 15, 2158–2166 (2013). https://doi.org/10.1039/C3GC40792F
T.P. Loh, S.K.W. Liung, K.L. Tan, L.L. Wei, Three component synthesis of b-amino carbonyl compounds using indium trichloride-catalyzed one-pot mannich-type reaction in water. Tetrahedron 56, 3227–3323 (2000). https://doi.org/10.1016/S0040-4020(00)00221-0
J. Mondal, A. Modak, A. Bhaumik, Highly efficient mesoporous base catalyzed Knoevenagel condensation of different aromatic aldehydes with malononitrile and subsequent noncatalytic Diels–Alder reactions. J. Mol. Catal. A 335, 236–241 (2011). https://doi.org/10.1016/j.molcata.2010.11.039
L. Hua, Z. Hong-yao, S. Hua-wu, Bismuth(III) chloride-catalyzed one-pot Mannich reaction: three-component synthesis of β-amino carbonyl compounds. Tetrahedron Lett. 50, 6858–6860 (2009). https://doi.org/10.1016/j.tetlet.2009.09.131
B.K. Ghosh, S. Hazra, B. Naik, N.N. Ghosh, Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 269, 371–378 (2015). https://doi.org/10.1016/j.powtec.2014.09.027
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anbu Anjugam Vandarkuzhali, S., Viswanathan, B., Pachamuthu, M.P. et al. Fine Copper Nanoparticles on Amine Functionalized SBA-15 as an Effective Catalyst for Mannich Reaction and Dye Reduction. J Inorg Organomet Polym 30, 359–368 (2020). https://doi.org/10.1007/s10904-019-01194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01194-0