Abstract
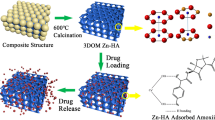
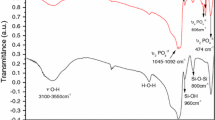
In this study, an efficient and robust metal organic framework (MOF)-based drug delivery system comprising high potential for slow release rate is introduced. Porous Fe-MIL-88B-NH2 nano-carrier, a series of flexible designed MOF materials, was synthesized and evaluated for slow released of alendronate (Alen). The Fe-MIL-88B-NH2 nano-carrier was synthesized by direct mixing of 2-aminoterephthalic acid and ferric chloride in the presence of pluronic F127 and acetic acid. Then, the surface of the nanoparticles was modified using β-cyclodextrin in order to increase the loading of Alen. After the loading process, the nano-drug carrier was encapsulated with hydroxyapatite (HAp) as a coating agent having the bone-like structure, which assists in lowering the drug release rate by decreasing the demand dosage. HAp is in high compatibility with Alen in terms of their positive impact on body bones. In order to confirm the synthesis of the nano-carrier system, scanning electron microscope, X-ray powder diffraction, Fourier transfer infrared, thermogravimetric analysis and BET surface analysis were used. Finally controlled released of Alen over 28 days was studied, which showed good results in comparison with previous systems.








Similar content being viewed by others
References
J.H. Tyman, Synthetic and Natural Phenols (Elsevier, New York, 1996)
J. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, S.T. Nguyen, J.T. Hupp, Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 (2009)
A. Dhakshinamoorthy, M. Opanasenko, J. Čejka, H. Garcia, Metal organic frameworks as solid catalysts in condensation reactions of carbonyl groups. Adv. Synth. Catal. 355, 247–268 (2013)
A. Dhakshinamoorthy, M. Opanasenko, J. Čejka, H. Garcia, Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 3, 2509–2540 (2013)
C.-H. Kuo, Y. Tang, L.-Y. Chou, B.T. Sneed, C.N. Brodsky, Z. Zhao, C.-K. Tsung, Yolk–shell nanocrystal@ ZIF-8 nanostructures for gas-phase heterogeneous catalysis with selectivity control. J. Am. Chem. Soc. 134, 14345–14348 (2012)
A.C. McKinlay, R.E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur, C. Serre, BioMOFs: metal–organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 49, 6260–6266 (2010)
A. Corma, State of the art and future challenges of zeolites as catalysts. J. Catal. 216, 298–312 (2003)
A.C. McKinlay, B. Xiao, D.S. Wragg, P.S. Wheatley, I.L. Megson, R.E. Morris, Exceptional behavior over the whole adsorption-storage-delivery cycle for NO in porous metal organic frameworks. J. Am. Chem. Soc. 130, 10440–10444 (2008)
B. Xiao, P.S. Wheatley, X. Zhao, A.J. Fletcher, S. Fox, A.G. Rossi, I.L. Megson, S. Bordiga, L. Regli, K.M. Thomas, High-capacity hydrogen and nitric oxide adsorption and storage in a metal-organic framework. J. Am. Chem. Soc. 129, 1203–1209 (2007)
S. Ma, H.-C. Zhou, Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 46, 44–53 (2010)
L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey, G.D. Weireld, Comparative study of hydrogen sulfide adsorption in the MIL-53 (Al, Cr, Fe), MIL-47 (V), MIL-100 (Cr), and MIL-101 (Cr) metal–organic frameworks at room temperature. J. Am. Chem. Soc. 131, 8775–8777 (2009)
M. Dincă, J.R. Long, Hydrogen storage in microporous metal–organic frameworks with exposed metal sites. Angew. Chem. Int. Ed. 47, 6766–6779 (2008)
W.J. Rieter, K.M. Taylor, H. An, W. Lin, W. Lin, Nanoscale metal-organic frameworks as potential multimodal contrast enhancing agents. Am. Chem. Soc. 128, 9024–9025 (2006)
K.M. Taylor, W.J. Rieter, W. Lin, Manganese-based nanoscale metal–organic frameworks for magnetic resonance imaging. Am. Chem. Soc. 130, 14358–14359 (2008)
Z.P. Xu, Q.H. Zeng, G.Q. Lu, A.B. Yu, Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 61, 1027–1040 (2006)
C.J. Murphy, A.M. Gole, J.W. Stone, P.N. Sisco, A.M. Alkilany, E.C. Goldsmith, S.C. Baxter, Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc. Chem. Res. 41, 1721–1730 (2008)
H. Deng, C.J. Doonan, H. Furukawa, R.B. Ferreira, J. Towne, C.B. Knobler, B. Wang, O.M. Yaghi, Multiple functional groups of varying ratios in metal-organic frameworks. Science 327, 846–850 (2010)
M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’keeffe, O.M. Yaghi, Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002)
B. Hoskins, R. Robson, Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI [4, 4′, 4″, 4‴-tetracyanotetraphenylmethane] BF4·xC6H5NO2. J. Am. Chem. Soc. 112, 1546–1554 (1990)
Z. Wang, S.M. Cohen, Postsynthetic covalent modification of a neutral metal-organic framework. J. Am. Chem. Soc. 129, 12368–12369 (2007)
C. Janiak, J.K. Vieth, MOFs, MILs and more: concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 34, 2366–2388 (2010)
M.T. Drake, B.L. Clarke, S. Khosla, Bisphosphonates: mechanism of action and role in clinical practice. In Mayo Clinic Proceedings (Elsevier, New York, 2008), pp. 1032–1045
G.A. Rodan, H.A. Fleisch, Bisphosphonates: mechanisms of action. J. Clin. Investig. 97, 2692 (1996)
H.-J. Moon, Y.-P. Yun, C.-W. Han, M.S. Kim, S.E. Kim, M.S. Bae, G.-T. Kim, Y.-S. Choi, E.-H. Hwang, J.W. Lee, Effect of heparin and alendronate coating on titanium surfaces on inhibition of osteoclast and enhancement of osteoblast function. Biochem. Biophys. Res. Commun. 413, 194–200 (2011)
K. Miladi, S. Sfar, H. Fessi, A. Elaissari, Drug carriers in osteoporosis: preparation, drug encapsulation and applications. Int. J. Pharm. 445, 181–195 (2013)
S. Cremers, S. Papapoulos, Pharmacology of bisphosphonates. Bone 49, 42–49 (2011)
J. Lin, Bisphosphonates: a review of their pharmacokinetic properties. Bone 18, 75–85 (1996)
J. Marshall, K. Rainsford, C. James, R. Hunt, A randomized controlled trial to assess alendronate-associated injury of the upper gastrointestinal tract. Aliment. Pharmacol. Ther. 14, 1451–1457 (2000)
F. Lanza, Bisphosphonate mucosal injury—the end of the story? Dig. Liver Dis. 35, 67–70 (2003)
L. Ochiuz, C. Grigoras, M. Popa, I. Stoleriu, C. Munteanu, D. Timofte, L. Profire, A.G. Grigoras, Alendronate-loaded modified drug delivery lipid particles intended for improved oral and topical administration. Molecules 21, 858 (2016)
J.H. Lee, I.H. Ko, S.-H. Jeon, J.-H. Chae, J.H. Chang, Micro-structured hydroxyapatite microspheres for local delivery of alendronate and BMP-2 carriers. Mater. Lett. 105, 136–139 (2013)
S. Tarafder, S. Bose, Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl. Mater. Interfaces 6, 9955–9965 (2014)
K. Miladi, S. Sfar, H. Fessi, A. Elaissari, Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 30, 391–396 (2015)
W. Hur, M. Park, J.Y. Lee, M.H. Kim, S.H. Lee, C.G. Park, S.-N. Kim, H.S. Min, H.J. Min, J.H. Chai, Bioabsorbable bone plates enabled with local, sustained delivery of alendronate for bone regeneration. J. Control. Release 222, 97–106 (2016)
E.M. Del Valle, Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
L. Rehmann, H. Yoshii, T. Furuta, Characteristics of modified β-cyclodextrin bound to cellulose powder. Starch-Stärke 55, 313–318 (2003)
A. Noomen, S. Hbaieb, H. Parrot-Lopez, R. Kalfat, H. Fessi, N. Amdouni, Y. Chevalier, Emulsions of β-cyclodextrins grafted to silicone for the transport of antifungal drugs. Mater. Sci. Eng. C 28, 705–715 (2008)
B. Martel, M. Weltrowski, D. Ruffin, M. Morcellet, Polycarboxylic acids as crosslinking agents for grafting cyclodextrins onto cotton and wool fabrics: study of the process parameters. J. Appl. Polym. Sci. 83, 1449–1456 (2002)
F. Macaev, V. Boldescu, Cyclodextrins in asymmetric and stereospecific synthesis. Symmetry 7, 1699–1720 (2015)
H. Tang, A.S. Sutherland, L.M. Osusky, Y. Li, J.F. Holzwarth, C. Bohne, Chiral recognition for the complexation dynamics of β-cyclodextrin with the enantiomers of 2-naphthyl-1-ethanol. Photochem. Photobiol. Sci. 13, 358–369 (2014)
L.R. Bordajandi, P. Korytár, J. de Boer, M.J. González, Enantiomeric separation of chiral polychlorinated biphenyls on β-cyclodextrin capillary columns by means of heart-cut multidimensional gas chromatography and comprehensive two-dimensional gas chromatography. Application to food samples. J. Sep. Sci. 28, 163–171 (2005)
G. Fang, M. Xu, F. Zeng, S. Wu, β-cyclodextrin as the vehicle for forming ratiometric mercury ion sensor usable in aqueous media, biological fluids, and live cells. Langmuir 26, 17764–17771 (2010)
D. Xiao, X. Zhou, H. Li, Y. Fu, K. Duan, X. Lu, X. Zheng, J. Weng, Fabrication of hollow hydroxyapatite particles assisted by small organic molecule and effect of microstructure on protein adsorption. J. Eur. Ceram. Soc. 35, 1971–1978 (2015)
Z. Li, T. Wen, Y. Su, X. Wei, C. He, D. Wang, Hollow hydroxyapatite spheres fabrication with three-dimensional hydrogel template. CrystEngComm 16, 4202–4209 (2014)
H. Zhou, J. Lee, Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 7, 2769–2781 (2011)
C.B. Danoux, D. Barbieri, H. Yuan, J.D. de Bruijn, C.A. van Blitterswijk, P. Habibovic, In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration. Biomatter 4, e27664 (2014)
S. Xu, J. Shi, D. Feng, L. Yang, S. Cao, Hollow hierarchical hydroxyapatite/Au/polyelectrolyte hybrid microparticles for multi-responsive drug delivery. J. Mater. Chem. B. 2, 6500–6507 (2014)
V. Orlovskii, V. Komlev, S.M. Barinov, Hydroxyapatite and hydroxyapatite-based ceramics. Inorg. Mater. 38, 973–984 (2002)
C.M. Kanno, R.L. Sanders, S.M. Flynn, G. Lessard, S.C. Myneni, Novel apatite-based sorbent for defluoridation: synthesis and sorption characteristics of nano-micro-crystalline hydroxyapatite-coated-limestone. Environ. Sci. Technol. 48, 5798–5807 (2014)
L.J. Cummings, Hydroxyapatite chromatography: purification strategies for recombinant proteins. Methods Enzymol. 541, 67–83 (2013)
S. Kano, A. Yamazaki, R. Otsuka, M. Ohgaki, M. Akao, H. Aoki, Application of hydroxyapatite-sol as drug carrier. Biomed. Mater. Eng. 4, 283–290 (1994)
K. Tomoda, H. Ariizumi, T. Nakaji, K. Makino, Hydroxyapatite particles as drug carriers for proteins. Colloids Surf. B 76, 226–235 (2010)
S.P. Victor, W. Paul, M. Jayabalan, C.P. Sharma, Supramolecular hydroxyapatite complexes as theranostic near-infrared luminescent drug carriers. CrystEngComm 16, 9033–9042 (2014)
M.-H. Pham, G.-T. Vuong, A.-T. Vu, T.-O. Do, Novel route to size-controlled Fe–MIL-88B–NH2 metal–organic framework nanocrystals. Langmuir 27, 15261–15267 (2011)
J. Kuljanin, I. Janković, J. Nedeljković, D. Prstojević, V. Marinković, Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe (III) ions. J. Pharm. Biomed. 28, 1215–1220 (2002)
J. Biernacka, K. Betlejewska-Kielak, J. Witowska-Jarosz, E. Kłosińska-Szmurło, A.P. Mazurek, Mass spectrometry and molecular modeling studies on the inclusion complexes between alendronate and β-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 78, 437–443 (2014)
Funding
Funding was provided by Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences (IR) and Shahrood University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Golmohamadpour, A., Bahramian, B., Shafiee, A. et al. Slow Released Delivery of Alendronate Using β-Cyclodextrine Modified Fe–MOF Encapsulated Porous Hydroxyapatite. J Inorg Organomet Polym 28, 1991–2000 (2018). https://doi.org/10.1007/s10904-018-0871-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0871-2