Abstract
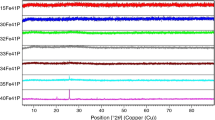
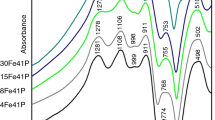
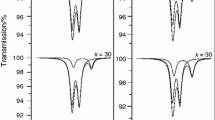
Ternary Na2O–Fe2O3–P2O5 (NFP) glasses with varying Na2O/Fe2O3, Na2O/P2O5, and Fe2O3/P2O5 ratios were prepared. The properties and crystallization tendencies were systemically investigated. It is shown that both density and chemical stability of the glass increase with Fe2O3. In contrast the Na2O/P2O5 ratio has little effect on the glass properties for a fixed Fe2O3 content. The crystallization behavior of the glasses was analyzed by DTA and XRD. Unlike Li2O–Fe2O3–P2O5 glasses NFP glasses were found to be stable against crystallization. 15Na2O–27Fe2O3–58P2O5 glass was found to have the highest chemical stability among the studied NFP samples; the influence of TiO2, ZrO2 on crystallization in this composition was studied. It is found that addition of 3.4 mol% TiO2 or 2.2 mol% ZrO2 had little effect on the crystallization behavior of this glass. However, when the amounts of TiO2 or ZrO2 were increased to 8.4 or 5.5 mol% respectively the glass readily devitrified. Furthermore the addition of fluorine (introduced by replacing Na2CO3 with NaF in the glass batch) leads to amorphous glasses which could be crystallized to form NaFeP2O7 upon controlled thermal treatment. With increasing NaF additions the activation emergy for crystallization decreased from 428 to 381 kJ/mol.






Similar content being viewed by others
References
R. Morena, J. Non-Cryst. Solids 263, 382 (2000)
J.C. Knowles, J. Mater. Chem. 13, 2395 (2003)
J.H. Campbell, T.I. Suratwala, J. Non-Cryst. Solids 263, 318 (2000)
C.S. Ray, X. Fang, M. Karabulut, G.K. Marasinghe, D.E. Day, J. Non-Cryst. Solids 249, 1 (1999)
R.K. Brow, J. Non-Cryst. Solids 263, 1 (2000)
T. Uchino, T. Yoko, J. Non-Cryst. Solids 263, 180 (2000)
X. Yu, D.E. Day, G.J. Long, R.K. Brow, J. Non-Cryst. Solids 215, 21 (1997)
B.C. Sales, L.A. Boatner, Science 226, 45 (1984)
I.W. Donald, B.L. Metcalfe, R.N.J. Taylor, J. Mater. Sci. 32, 5851 (1997)
M.G. Mesko, D.E. Day, J. Nucl. Mater. 273, 27 (1999)
Y. M. Moustafa, K. El-Egili, H. Doweidar, I. Abbas, Phys. B 353, 82 (2004)
J.L. Shaw, A.C. Wright, R.N. Sinclair, G.K. Marasinghe, D. Holland, M.R. Lees, C.R. Scales, J. Non-Cryst. Solids 345, 245 (2004)
M.M. El-Desoky, K. Tahoon, M.Y. Hassaan, Mater. Chem. Phys. 69, 180 (2001)
L. Ma, R.K. Brow, A. Choudhury, J. Non-Cryst. Solids 402, 64 (2014)
P. Stoch, M. Ciecinska, A. Stoch, J. Non-Cryst. Solids 117, 197 (2014)
T. Honma, A. Sato, N. Ito, T. Togashi, K. Shinozaki, T. Komatsu, J. Non-Cryst. Solids 404, 26 (2014)
X. Fang, C.S. Ray, G.K. Marasinghe, D.E. Day, J. Non-Cryst. Solids 263, 293 (2000)
A.J. Parsons, C.D. Rudd, J. Non-Cryst. Solids 354, 4661 (2008)
A. Moguš-Milanković, A. Gajović, A. Šantić, D.E. Day, J. Non-Cryst. Solids 289, 204 (2001)
P.A. Bingham, R.J. Hand, O.M. Hannant, S.D. Forder, S.H. Kilcoyne, J. Non-Cryst. Solids 355, 1526 (2009)
A. Moguš-Milanković, B. Pivac, K. Furic, D.E. Day, Phys. Chem. Chem. Phys. 38, 74 (1997)
M. Guignard, L. Cormier, V. Montouillout, N. Menguy, D. Massiot, J. Non-Cryst. Solids 356, 1368 (2010)
M. Mirkazemi, V.K. Marghussian, A. Beitollahi, S.X. Dou, D. Wexler, K. Konstantinov, Ceram. Intern. 33, 463 (2007)
G.A. Khater, M.H. Idris, Ceram. Intern. 35, 69 (2009)
A.W.A. El-Shennawi, M.M. Morsi, S.A.M. Abdel-Hameed, J. Eur. Ceram. Soc. 27, 1829 (2007)
A. Marotta, S. Saiello, F. Branda, A. Buri, J. Mater. Sci. 17, 105 (1982)
Acknowledgments
This work was financially supported by the Opening Projects Foundation of the State Key Laboratory of Crystal Material, Shandong University. We thank Prof. Russell Hand of the University of Sheffield, UK for commenting on the manuscript and helping with English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Liu, H., Zhang, W. et al. Properties and Crystallization Behavior of Sodium Iron Phosphate Glasses. J Inorg Organomet Polym 27, 159–164 (2017). https://doi.org/10.1007/s10904-016-0458-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0458-8