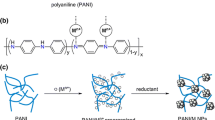
Abstract
The present study summarizes the preparation of p-toluenesulfonic acid (p-TsOH) doped PANI/M (M = Pt, Pd, Ag, and Ni) nanohybrid electrodes and the activity for hydrogen peroxide generation using the electrodes. The activity depended on the kinds of the metals. The improvement of durability was suggested in the case of PANI/Ag and PANI/Ni nanohybrids as compared to that of PANI. The UV–Vis absorption spectra for the electrode films gave the information of the redox behavior of PANI before and after the reactions. The obtained results are considered to be valuable for the sterilization use.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Active oxygen is useful for sterilizing the bacteria and microbe [1]. Several methods to generate active oxygen or related species have been developed. Exposing photocatalysts such as TiO2 to light is one of the most efficient methods [2, 3]. Addition of polyaniline (PANI) powder to pure water was also reported to form active oxygen species [4]. Another promising method is electrochemical reduction of molecular oxygen on a cathode electrode [5]. As the examples of the electrochemical reduction, some reports described the methods using electrode catalysts with redox-active conductive polymer PANI [6–8] and the hybrid of anthraquinone and polypyrrole [9, 10]. In the case of using PANI as an electrode catalyst, the mechanism to generate active oxygen species is proposed that PANI is reduced on a cathode electrode, then the reduced PANI is oxidized by molecular oxygen, resulting in a superoxide anion radical (Fig. 1a, where the quantity of superoxide anion radical is estimated by quantifying the hydrogen peroxide which is considered to be derived from the disproportionation) [6]. Such a redox catalytic cycle of PANI allows continuous generation of the superoxide anion radical. Generation of superoxide anion radical in alkaline aqueous solution was also reported utilizing a PANI electrode [8]. It was demonstrated that the PANI electrode hybridized with Dawson-type hetero polyanion can reduce molecular oxygen efficiently to generate hydrogen peroxide [11]. On the other hand, the synthesis, evaluation and catalytic applications of PANI/transition metal (M) nanohybrids have been researched in the previous papers [12–27]. Especially, it has been reported that the catalyst consisting of poly(2-methoxyaniline-5-sulfonic acid) (PMAS) with Au nanoparticles is useful to the aerobic oxidation of alcohols and amines [21, 23–25]. The investigation of these reactions by UV–Vis absorption spectroscopy suggested that PMAS performs as a redox mediator between molecular oxygen and Au nanoparticles as shown in Fig. 1b [21, 24, 25]. In such backgrounds, we envisaged that use of a cathode electrode as an electron source instead of the alcohols and amines would give an efficient catalytic cycle with the PANI/M nanohybrids for generation of active oxygen species. The PANI/M (represented by Pt) nanohybrids are well known as useful electrode catalysts for oxygen reduction in fuel cell [28–34], but where molecular oxygen is reduced to water under the acidic conditions and the investigation has been less-performed from the viewpoint of the generation of active oxygen species which arises as intermediates. Such acidic conditions are not desirable in case of the antibacterial use from the environmental point of view. Here, we report the preparation of the electrodes supported by the proton doped PANI/M (M = Pd, Pt, Ag, and Ni) nanohybrids and their evaluation for the hydrogen peroxide generation (Fig. 1c), and found the characteristic behaviors depending on the kind of metals by UV–Vis absorption spectroscopy.
2 Experimental
2.1 Materials and Method
All reagents were purchased from commercial sources and used as received without any further purification. PANI was purchased from Aldrich (emeraldine base, Mw = 7,500–15,000). UV–Vis absorption spectra were obtained on a SHIMADZU UV–Vis–NIR SPECTROMETER UV-3150.
2.2 Synthesis of the PANI/M Nanohybrids
The synthetic procedure and characterization of PANI/M (M = Pd and Pt) (M: 10 wt% to PANI) nanohybrids were reported previously [27]. The PANI/M (M = Ag and Ni) (M: 10 wt% to PANI) nanohybrids were synthesized by the similar method as follows: PANI (500 mg) was dissolved in THF (150 mL) by sonication. To the stirred solution was dropwise added a solution of AgNO3 (78.7 mg, 0.464 mmol) or NiCl2 (110.4 mg, 0.852 mmol) in THF/H2O (20/5 mL) at room temperature over ~30 min. The reaction mixture was stirred at room temperature for ~1 h to form suspension. A solution of NaBH4 (10 equivalents to the Mn+) in ethanol (25 mL) was dropwise added to the suspension over ~30 min. The mixture was stirred at room temperature for ~1 h (the mixture was stirred at 60 °C for 3 h for the PANI/Ni nanohybrid). The solvent was evaporated, and then H2O (~100 mL) was added. The suspension was sonicated. The mixture was filtered, and the residue was washed with H2O and dried in vacuo to give the PANI/M (M = Ag and Ni) nanohybrids (400–500 mg).
2.3 Preparation of the Electrodes
PANI (150 mg) or the PANI/M nanohybrids (150 mg) were dissolved in N-methyl-2-pyrrolidone (NMP) (10 g). p-Toluenesulfonic acid (p-TsOH) was added as a dopant (1 equivalent to the aniline unit of PANI). A 10 wt% solution (3.0 g) of dipentaerythritol hexaacrylate/2-butoxyethanol was added to the PANI/M nanohybrid solution as a binder. The mixtures were spin-coated (500 rpm, 10 s.) on ITO/glass substrate (5 cm × 5 cm) and dried at 150 °C for 1 min. The dried film was cured by UV irradiation. The cut film (5 cm × 2.5 cm) was used as an electrode.
2.4 Generation of Hydrogen Peroxide
The equipment for the hydrogen peroxide generation is shown in Fig. 2. The content of hydrogen peroxide was measured by using semi-quantitative test strip (QuantofixTM Peroxide 25, purchased from MACHEREY–NAGEL). Physiological saline (50 g) was used as a solvent. The applied voltage was fixed to 2.5 V. The content of hydrogen peroxide was measured after applying voltage for 6 h. Then, the electrode was picked out from the aqueous solution and dried in vacuo at room temperature for more than 12 h. And this operation was repeated to test the repetition durability.
3 Results and Discussions
3.1 Synthesis
The PANI/M (M = Pt and Pd) (M: 10 wt% to PANI) nanohybrids were synthesized by the developed template method, which includes two steps (1) complexation of metal salts on the PANI template and (2) reduction of metal salts with NaBH4. Their synthetic procedure and characterization are described in the previous report [27]. The other PANI/M (M = Ag and Ni) nanohybrids (M: 10 wt% to PANI) were synthesized by the similar method.
3.2 Electrode
Proton doping was performed by adding one equivalent of p-TsOH to the purple NMP solution of PANI/M (M = Pd, Pt, Ag, and Ni) nanohybrids. The color of the solutions changed to green, which is characteristic of the emeraldine salt. A photo curable dipentaerythritol hexaacrylate was used as a binder. The film was formed by spin coating of the solution on ITO electrode and cured with UV irradiation. The thickness of thus-obtained films was 190–230 nm. Figure 3 shows the UV–Vis absorption spectra for each electrode film. The absorption peaks in the range of 300–350 nm and 450–500 nm are attributable to the π–π* transition of the benzene ring and polaron absorption, respectively [35].
3.3 Hydrogen Peroxide Generation
Hydrogen peroxide was quantified instead of superoxide anion radical in the present study because superoxide anion radical disproportionates to result in hydrogen peroxide [6]. Table 1 shows the concentration of hydrogen peroxide for each electrode in physiological saline. At first, the generation of hydrogen peroxide was examined by using the p-TsOH doped PANI electrode. After applying voltage (2.5 V) for 6 h, the concentration of hydrogen peroxide in physiological saline (50 mL) was 2.0 ppm. In order to investigate the durability, this experiment was repeated five times. As a result, the concentration of hydrogen peroxide decreased gradually and became 0 ppm in the fifth cycle. And the color of electrode film was changed from green to purple. In the case of the p-TsOH doped PANI/M (M = Pd and Pt) nanohybrids, the concentration of hydrogen peroxide was 3.5 ppm in the first cycle, which is larger than that generated by using p-TsOH doped PANI in the first cycle. However, the concentration of hydrogen peroxide was decreased to 0 ppm in the third cycle in the case of the p-TsOH doped PANI/M (M = Pd and Pt) nanohybrids. On the other hands, the p-TsOH doped PANI/M (M = Ag and Ni) hybrids generated 2 ppm of hydrogen peroxide in the first cycle, which is almost comparable to that generated by using p-TsOH doped PANI. The concentration of hydrogen peroxide was maintained even after the fifth cycle in the case of the p-TsOH doped PANI/M (M = Ag and Ni) nanohybrids.
In order to investigate the state of PANI on the electrode films after the fifth cycle of the experiments, the UV–Vis absorption spectra were measured for each electrode (Fig. 4). In the case of the PANI/Pt nanohybrids, the peak around 450 nm decreased as compared to that before the reactions. Dedoping of p-TsOH by seeping it into the aqueous solution and/or reduction of PANI might occur. The same reasons are considered for decreasing of the activity in hydrogen peroxide generation for the PANI electrode as possibilities because the slight decreasing of the polaron absorption was observed in the spectrum for PANI. In the case of the PANI/Pd nanohybrid, the peak around 450 nm slightly decreased and the broad peak around 540 nm slightly increased, which was not observed before the reactions. This spectrum is relatively similar to that for the oxidized form of PANI [36]. The electrode reduction after oxidation of PANI by molecular oxygen might be inefficient. In the case of PANI/Ag nanohybrid, the absorption spectra were comparatively similar before and after the examination. In fact, the generation of hydrogen peroxide was maintained after the fifth cycle. On the other hand, the spectral behavior of PANI/Ni one was different although the generation of hydrogen peroxide was maintained. In the spectrum after the fifth cycle, the absorption more than 400 nm became flattened, but the very broad absorption from 400 to 750 nm is still present. The peak around 330 nm is attributable to π–π* transition. This spectrum might suggest the reduced form of PANI [36], indicating that the reduction of PANI on the electrode proceeded smoothly in the PANI/Ni nanohybrid system.
4 Conclusion
The present study summarizes the preparation of p-TsOH doped PANI/M (M = Pt, Pd, Ag, and Ni) nanohybrid electrodes and the investigation of the activity for hydrogen peroxide generation using the electrodes. The activity depended on the kinds of the metals. The improvement of durability was suggested in the case of PANI/Ag and PANI/Ni nanohybrids as compared to that of PANI. The UV–Vis absorption spectra for electrode films gave the information of the redox behavior of PANI before and after the reactions. The obtained results are considered to be valuable for the sterilization use.
References
E. Cabiscol, J. Tamarit, J. Ros, Int. Microbiol. 3, 3 (2000)
K. Hashimoto, H. Irie, A. Fujishima, Jpn. J. Appl. Phys. 44, 8269 (2005)
M. Pelaez, N.T. Nolan, S.C. Pillai, M.K. Seery, P. Falaras, A.G. Kontos, P.S.M. Dunlop, J.W.J. Hamilton, J.A. Byrne, K. O’Shea, M.H. Entezari, D.D. Dionysiou, Appl. Catal. B 125, 331 (2012)
S. Otsuka, K. Saito, K. Morita, Chem. Lett. 25, 615 (1996)
A. Kraft, Platinum Metals Rev. 52, 177 (2008)
N. Kawashima, M. Takamatsu, K. Morita, Colloids Surf. B 11, 297 (1998)
H. Ono, T. Okajima, F. Matsumoto, T. Oritani, A. Shimizu, M. Takahashi, T. Ohsaka, Electrochemistry 69, 953 (2001)
N. Ohki, S. Uesugi, K. Murayama, F. Matsumoto, N. Koura, T. Okajima, T. Ohsaka, Electrochemistry 70, 23 (2002)
P. Manisankar, A. Gomathi, J. Mol. Catal. A 232, 45 (2005)
G. Zhang, F. Yang, W. Yang, React. Funct. Polym. 67, 1008 (2007)
D.G. Shchukin, D.V. Sviridov, Electrochem. Commun. 4, 402 (2002)
T. Hirao, M. Higuchi, B. Hatano, I. Ikeda, Tetrahedron Lett. 36, 5925 (1995)
M. Higuchi, D. Imoda, T. Hirao, Macromolecules 29, 8277 (1996)
T. Hirao, S. Yamaguchi, S. Fukuhara, Synth. Met. 106, 67 (1999)
T. Hirao, S. Yamaguchi, S. Fukuhara, Tetrahedron Lett. 40, 3009 (1999)
T. Hirao, S. Fukuhara, Y. Otomaru, T. Moriuchi, Synth. Met. 123, 373 (2001)
X. Shen, T. Moriuchi, T. Hirao, Tetrahedron Lett. 45, 4733 (2004)
T. Amaya, D. Saio, T. Hirao, Tetrahedron Lett. 48, 2729 (2007)
T. Amaya, D. Saio, T. Hirao, Macromol. Symp. 270, 88 (2008)
D. Saio, T. Amaya, T. Hirao, J. Inorg. Organomet. Polym. 19, 79 (2009)
D. Saio, T. Amaya, T. Hirao, Adv. Synth. Catal. 352, 2177 (2010)
T. Amaya, T. Hirao, Synlett 435 (2011)
T. Amaya, T. Ito, T. Hirao, Heterocycles 86, 927 (2012)
T. Amaya, T. Ito, Y. Inada, D. Saio, T. Hirao, Tetrahedron Lett. 53, 6144 (2012)
T. Amaya, T. Ito, T. Hirao, Tetrahedron Lett. 54, 2409 (2013)
T. Isaji, T. Amaya, Y. Inada, M. Abe, T. Hirao, Bull. Chem. Soc. Jpn 87, 1130 (2014)
T. Amaya, T. Isaji, M. Abe, T. Hirao, J. Inorg. Organomet. Polym. 25, 145 (2015)
H. Nakano, Y. Tachibana, S. Kuwabata, Electrochim. Acta 50, 749 (2004)
A. Kongkanand, S. Kuwabata, J. Phys. Chem. B 109, 23190 (2005)
V.G. Khomenko, V.Z. Barsukov, A.S. Katashinskii, Electrochim. Acta 50, 1675 (2005)
A. Drelinkiewicz, A. Zięba, J.W. Sobczak, M. Bonarowska, Z. Karpiński, A. Waksmundzka-Gόra, J. Stejskal, React. Funct. Polym. 69, 630 (2009)
A. Morozan, B. Jousselme, S. Palacin, Energy Environ. Sci. 4, 1238 (2011)
S. Chen, Z. Wei, X. Qi, L. Dong, Y. Guo, L. Wan, Z. Shao, L. Li, J. Am. Chem. Soc. 134, 13252 (2012)
J. Zhang, D. He, H. Su, X. Chen, M. Pan, S. Mu, J. Mater. Chem. A 2, 1242 (2014)
Y. Xia, J.M. Wiesinger, A.G. MacDiarmid, Chem. Mater. 7, 443 (1995)
E.T. Kang, K.G. Neoh, K.L. Tan, Prog. Polym. Sci. 23, 277 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isaji, T., Abe, M., Amaya, T. et al. Hydrogen Peroxide Generation Using Polyaniline/Transition Metal Nanohybrid Electrodes. J Inorg Organomet Polym 25, 855–859 (2015). https://doi.org/10.1007/s10904-015-0172-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0172-y