Abstract
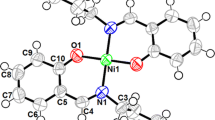
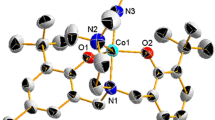
New Schiff base (HL) ligand is prepared via condensation of 2-aminophenol and 4-(dimethylamino) cinnamaldehyde. The cobalt complex with the formula of [Co(L)2(MePy)2]PF6 is prepared in good yield from the reaction of the ligand with cobalt(II) acetate. The compounds were characterized by the analytical and spectroscopic methods. The ligand (HL) behaves as a bidentate ligand and coordinates to the cobalt ions via the nitrogen and oxygen atoms with mononuclear structure. The ligand (HL) has been structurally characterized by X-ray crystallography. There is O–H···N–H bond linking the molecules together. FT-IR spectra show that HL is coordinated to the cobalt ion in a mono-negative bidentate manner with ON donor sites of the azomethene N and deprotonated phenolic-OH. There are two methylpyridine molecules apically coordinated to Co(III) ion in octahedral environment. Electrochemical properties were investigated as metal centres in the dimethylformamide solvents. The Co(III) complex exhibit both irreversible reductive (CoIII/CoII; Epc, −1.24 V) and oxidative (CoII/CoIII; Epa, +0.79 V, respectively) responses in cyclic voltammetry. Thin film of nano-sized [M(L)2(MePy)2]PF6 complex was formed by surface layer-by-layer chemical deposition method. The formation of uniform thin films of nano-crystalline metal complex is dependent on metal and ligand concentrations.











Similar content being viewed by others
References
B. Kurzak, A. Kamecka, K. Bogusz, J. Jezierska, Polyhedron 26, 4345–4350 (2007)
P.M. Reddy, A.V.S.S. Prasad, K. Shanker, V. Ravinder, Spectrochim. Acta A 68, 1000–1006 (2007)
P.M. Reddy, K. Shanker, R. Rohini, M. Sarangapani, V. Ravinder, Spectrochim. Acta A 70, 1231–1237 (2008)
P.M. Reddy, A.V.S.S. Prasad, R. Rohini, V. Ravinder, Spectrochim. Acta A 70, 704–712 (2008)
B. Retcher, J.S. Costa, J. Tang, R. Hage, P. Gamez, J. Reedijk, J. Mol. Catal. A: Chem 286, 1–5 (2008)
M. Ashok, A.V.S.S. Prasad, P.M. Reddy, V. Ravinder, Spectrochim. Acta A 72, 204–208 (2009)
K. Shanker, R. Rohini, V. Ravinder, P.M. Reddy, Y.P. Ho, Spectrochim. Acta A 73, 205–211 (2009)
S.A. Patil, V.H. Naik, A.D. Kulkarni, P.S. Badami, Spectrochim. Acta A 75, 347–354 (2010)
R. Luque, S.K. Badamali, J.H. Clark, M. Fleming, D.J. Macquarrie, Appl. Catal. A: Gen. 341, 154–159 (2008)
L.A. Saghatforoush, R. Mehdizadeh, F. Chalabian, Transit. Met. Chem. 35, 903–910 (2010)
H.H. Nalwa, Handbook of nanostructured materials and nanotechnology, vol. 1–5 (Academic Press, Boston, 2000)
M. H. Habibi, M. Nasr-Esfahani, Int. J. Photoenergy (2008) 628713
M.H. Habibi, N. Talebian, Thin Solid Films 515, 1461–1469 (2006)
M.H. Habibi, N. Talebian, J.H. Choi, Dyes Pigments 73, 103–110 (2007)
M.H. Habibi, N. Talebian, Dyes Pigments 73, 186–194 (2007)
T. Higashi, Shape program for absorption (Rigaku Corporation, Tokyo, 2005)
M.C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G.L. Cascarano, L. De Caro, C. Giacovazzo, G. Polidori, R. Spagna, J. Appl. Cryst. 38, 381 (2005)
G. M. Sheldrich, SHELXL 97. University of Gottingen, Gottingen (1997)
T.T. Wang, J.M. Xie, C.K. Xia, Y.L. Wu, J.J. Jing, Anorg. Allg. Chem. 636, 1580–1588 (2010)
F.M. Hwang, H.Y. Chen, P.S. Chen, C.S. Liu, Y.C. Chi, C.F. Shu, F.L. Wu, P.T. Chou, S.M. Peng, G.H. Lee, Inorg. Chem. 44, 1344–1349 (2005)
S. Naskar, S. Biswas, D. Mishr, B. Adhikary, L.R. Falvello, T. Soler, C.H. Schwalb, S.K. Chattopadhyay, Inorg. Chim. Acta 357, 4257–4262 (2004)
M.A. Baldo, M.E. Thompson, S.R. Forest, Nature 403, 750–756 (2000)
S. Biswas, K. Mitra, C.H. Schwalbe, C.R. Lucas, S.K. Chattopadhyay, B. Adhikary, Inorg. Chim. Acta 358, 2473–2478 (2005)
Acknowledgment
The authors wish to thank the University of Isfahan for financially supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habibi, M.H., Shojaee, E., Yamane, Y. et al. Synthesis, Spectroscopic Studies, Crystal Structure and Electrochemical Properties of New Cobalt(III) Complex Derived from 2-Aminophenol and 4-(Dimethylamino)Cinnamaldehyde: Nano-Sized Complex Thin Film Formation via Surface Layer-by-Layer Chemical Deposition Method. J Inorg Organomet Polym 22, 190–195 (2012). https://doi.org/10.1007/s10904-011-9535-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9535-1