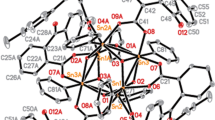
Two types of diorganotins [R2Sn(OCOC5H3N-3-Br-5)2] n and {[R2Sn(OH2)(OCOC5H3N-3-Br-5)2]2} n (R= Me, n-Bu, Ph, n-Oc), are prepared from 5-Br-omonicotinic acid and diorganotin oxides. All the compounds, 1–8, are characterized by elemental analysis as well as IR and 1H-NMR spectroscopy. The crystal structures of [R2Sn(OCOC5H3N-3-Br-5)2] n (2) and {[R2Sn(OH2)(OCOC5H3N-3-Br-5)2]2} n (8) were determined by single crystal X-ray diffraction. In compound 2, each carboxylate moiety of 5-Br-omonicotinic acid is involved in coordination to one Sn atom via two O-atoms, and the N-atom of one pyridine-ring coordinates to the neighboring Sn atom which leads to a polymeric chain. And the N-atom of the other pyridine-ring is dissociative. In compound 8, the compound proves to be dinuclear macrocyclic compounds with 5-Br-omonicotinic acid bridging the adjacent tin atoms with a 12-member ring. The hydrogen bonds ( \(\hbox{N}\ldots\hbox{H}-\hbox{O}\)) are observed in the compound 8. These intermolecular hydrogen bonds form another ring, and lead to a polymeric chain in the lattice at the same time.







Similar content being viewed by others
References
Casas J. S., Castineiras A., Couce M. D., Jorge M. L., Russo U., Sanchez A., Seoane R., Varela J. M. (2000) Appl. Organometal. Chem. 14:421
Li J., Ma Y., Liu R., Li J. (2001) Appl. Organometal. Chem. 15:227
K. C. Molloy, K. Quill, and I. W. Nowell, J. Chem. Soc., Dalton Trans. 101 (1987)
Gielen M. (2002) Appl. Organomet. Chem. 16:481
Zubita J. A. and Zuckerman J. J. (1987) Inorg. Chem. 24:251
Sandhu G. K., Gupta R., Sandhu S. S., and Parish R. V. (1985). Polyhedron 4:81
Sandhu G. K., Gupta R., Sandhu S. S., Parish R. V., and Brown K. (1985). J. Organomet. Chem. 279:372
Lochhart T. P. and Davidson F. (1987). Organometallics 6:2471
Nowell I. W., Brooks J. S., Beech G., and Hill R. (1983). J. Organomet. Chem. 244:119
Khan M. I., Baloch M. K., and Ashfaq M. (2004) J. Organoment. Chem. 689:4584
Parulekar C. S., Jain X. K., Das T. K., Gupta A. R., Hoskins B. F., and Tiekink E. R. T. (1989) J. Organomental. Chem. 372:193
Xie Q. L., Xu X. H., Wang H. G., Yao X. K., Zhang R. J. Z. G., and Hu J. M. (1991) Acta. Chim. Sinica 49:1085
Lockhart T. P., and Davidson F. (1987) Organometallics 6:2471
Vatsa C., Jain V. K., Kesavadas T., and Tiekink E. R. T. (1991) J. Organomet. Chem. 410:135
Yin H. D., Wang C. H., Wang Y., Ma C. L., and Wang D. Q. (2004) J. Organomet. Chem. 689:246
Bellamy L. J. (1975). The Infra-red Spectra of Complex Molecules. 3rd ed., John Wiley, New York
Ye Y., Zeng Q., and Liu L. Z. (2001) Synth. Commun. 31:2373
Yin H. D., Wang C. H., Wang Y., Zhu D. Z., and Ma C. L. (2002) Chim. J. Chem. 19:1146
Harrison P. G., and Philips R. C. (1979) J. Organomet. Chem. 182:37
Acknowledgments
We acknowledged the National Natural Foundation of the Peoples Republic of China (Grant #20271025), Shandong Province Science Foundation (Grant #L2003B01), and State Key Laboratory of Crystal Materials of Shandong University, Peoples Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10904-007-9116-5
Rights and permissions
About this article
Cite this article
Gao, Z., Yin, H. & Sun, L. Synthesis of Diorganotin Esters of 5-Bromonicotinic Acid: X-ray Crystal Structure of Polymeric 5-Bromonicotinatodiorganotin. J Inorg Organomet Polym 16, 83–89 (2006). https://doi.org/10.1007/s10904-006-9026-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-006-9026-y