Abstract
Six refugee screening sites collaborated to estimate the prevalence of hepatitis C virus (HCV) antibodies among newly arrived refugees in the United States from 2010 to 2017, identify demographic characteristics associated with HCV antibody positivity, and estimate missed HCV antibody-positive adults among unscreened refugees. We utilized a cross-sectional study to examine HCV prevalence among refugees (N = 144,752). A predictive logistic regression model was constructed to determine the effectiveness of current screening practices at identifying cases. The prevalence of HCV antibodies among the 64,703 refugees screened was 1.6%. Refugees from Burundi (5.4%), Moldova (3.8%), Democratic Republic of Congo (3.2%), Burma (2.8%), and Ukraine (2.0%) had the highest positivity among refugee arrivals. An estimated 498 (0.7%) cases of HCV antibody positivity were missed among 67,787 unscreened adults. The domestic medical examination represents an opportunity to screen all adult refugees for HCV to ensure timely diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis C Epidemiologic Overview
Hepatitis C virus (HCV) infects the liver and can lead to cirrhosis, hepatocellular carcinoma (HCC), the need for liver transplantation, and death. Global prevalence estimates of chronic hepatitis C infection during 2015 were approximately 1%, with variation between and within countries [1]. The prevalence of hepatitis C antibodies in the adult United States population during 2013–2016 was estimated at 1.7% (95% CI: 1.4 − 2.0%) [2]. HCV infection is diagnosed via testing for anti-HCV antibodies followed by a nucleic acid test for HCV ribonucleic acid (RNA) to confirm chronic infection in those who tested positive for anti-HCV antibodies. It is important to test for hepatitis C infection as the majority of infected people develop chronic viremia, most people do not experience symptoms or symptoms are nonspecific, and 90% of people can be cured with treatment in 8 to 12 weeks [3].
Refugee Resettlement in the United States and the Domestic Medical Examination
There were 600,898 refugees who arrived in the United States between 2010 and 2020 [4]. The Centers for Disease Control and Prevention (CDC) recommends that all newly arrived refugees receive a domestic medical examination (DME) within 30 to 90 days of arrival to the United States [5]. This comprehensive examination screens for infectious and non-communicable diseases and serves as a key mechanism for connecting refugees with routine and specialty care. Surveillance data from the DME provides an overview of the prevalence of a broad range of conditions likely associated with health status before resettlement in the United States.
From 2010 to 2011, CDC recommended hepatitis C screening during the DME for those with risk factors such as injection and intranasal drug use, chronic hemodialysis, HIV infection, signs or symptoms of liver disease, household contact with someone infected with HCV, or history of female genital mutilation or cutting [6]. Starting in 2012, hepatitis C screening was also recommended for refugees born between 1945 and 1965. Additionally, the guidelines stated that it was reasonable to screen all adults (≥ 18 years of age) who originated from or had lived in countries with high-moderate (2–5%) or high (≥ 5%) hepatitis C prevalence. Hepatitis C screening is not routinely recommended for children < 18 years old unless they have risk factors. In 2020, the CDC updated its hepatitis C screening guidelines for newly arrived refugees [6]. This update included universal hepatitis C screening for all new adult arrivals (≥ 18 years of age). Hepatitis C screening is also recommended for all pregnant people during each pregnancy, unaccompanied refugee minors, children with risk factors, and children born to mothers with hepatitis C.
Hepatitis C Prevalence and Risk Factors Among Refugees
A systematic review on the prevalence of hepatitis C antibodies among immigrants and refugees estimated an overall prevalence of 19 per 1,000 individuals (range: 14–27) [7]. Country of origin-based estimates of hepatitis C prevalence in refugees currently residing in the United States are sparse and inconsistent. Recent estimates for hepatitis C antibody prevalence among Somali refugees range from 8.5 to 91 per 1,000 [8, 9]. Chronic HCV prevalence was 5.1 per 1,000 (range: 0–18) for Hmong people in a camp in Laos compared to 72.3 per 1,000 (range: 52–93) among Hmong refugees in Thailand [8].
In regions with high endemicity, most infection results from iatrogenic exposure, such as contaminated needles, medical procedures, or receipt of unscreened contaminated blood products [7]. Previous research on refugees arriving in the United Kingdom indicates a higher odds of HCV infection among those ≥ 50 years (6.71, 2.67–16.87, p < 0.001) compared to those 15–24 years, as well as increased odds of HCV infection among those with a history of blood transfusion (5.19, 1.70–15.88, p = 0.004) [10]. To better estimate the prevalence of hepatitis C, six refugee screening sites collaborated to evaluate antibody screening results at the DME.
Study Objectives
The three main study objectives were to (1) estimate the prevalence of hepatitis C antibodies among newly arrived refugees seen at six sites in the United States from 2010 to 2017, (2) identify demographic correlates of hepatitis C antibody positivity, including country of origin, sex, and age, and (3) develop a predictive statistical model of hepatitis C antibody positivity using these demographic correlates to estimate missed cases of hepatitis C antibody positivity among unscreened refugees in the study population. Results could be used to inform more targeted screening guidelines. Furthermore, results could be used to estimate the number of additional cases of antibody positivity that could have been identified using CDC’s updated hepatitis C screening guidelines.
Methods
Study Design
A retrospective cross-sectional study examined hepatitis C screening and prevalence among newly arrived refugees to the United States between January 1, 2010, and December 31, 2017.
Setting
Six sites contributed data for the analysis, including four state refugee health programs and two clinics that perform the DME. Partners included the state refugee health programs in Colorado, Minnesota, Texas, and Washington, and refugee health clinics in Denver, Colorado and Philadelphia, Pennsylvania.
Population
The analysis included refugees, asylees, Cuban/Haitian entrants, Amerasians, Special Immigrant Visa holders, unaccompanied refugee minors, and certified victims of human trafficking who arrived between 2010 and 2017 at one of the six participating sites (N = 144,905). In this analysis, the people arriving to the United States under the visa statuses listed previously are collectively referred to as “refugees”. Refugees screened more than one year after their U.S. arrival date were excluded from the analysis. The results submitted from the participating six sites represented approximately 27.6% of all refugees arriving to the United States from 2010 to 2017 [4].
Variables
Demographic data included nationality, WHO region of origin (Africa, the Americas, Eastern Mediterranean, Europe, South-East Asia, and the Western Pacific), sex (male or female), and age at the DME [11]. Five sites provided data on an individual’s nationality, while one site could only provide the country of birth and the country of departure. In this case, nationality was extrapolated using an algorithm that combined ethnicity, language, country of birth, and country of departure. A categorical variable was derived for the prediction model, containing individual categories for the 14 countries with the largest number of refugees and the rest combined into one “other” category as outlined in Table 1. Refugees with the missing country of departure, country of birth, and nationality were excluded from the analysis. Data were excluded when the country of departure, country of birth, or nationality were listed as the United States. Age categories were defined as 0–4 years, 5–12 years, 13–17 years, 18–44 years, 45–64 years, and ≥ 65 years. In the prediction model, the 18-44-year age group was divided into different categories (18–23, 24–29, 30–35, and 36–44 years).
Screening for hepatitis C antibodies was defined as receiving laboratory screening for hepatitis C antibodies at the DME. Positive hepatitis C antibodies were defined as the presence of hepatitis C antibodies in laboratory evaluation performed at the DME. Indeterminate hepatitis C antibody results were excluded from prevalence and prevalence ratio analyses.
Data Source
Demographic and screening data were obtained from the individual site’s refugee health screening databases for the four refugee state health programs or electronic medical records for the two clinics. Data including personally identifiable information, such as name and date of birth, were removed at the host sites and combined for analysis.
Statistical Analysis
Descriptive statistics were conducted. Chi-squared tests were calculated for univariate analysis of baseline data. We performed a multiple binary logistic regression to calculate prevalence ratios and 95% confidence intervals (95% CI) to identify demographic factors associated with hepatitis C antibodies (WHO region of origin, sex, age). We evaluated the regression model for significant interactions, and the number of variables was reduced using backward elimination and likelihood ratio tests to determine the best model. Region of origin was used for the regression model due to smaller sample sizes within countries of origin. We used a multiple binary logistic regression model to estimate the number of cases of hepatitis C antibody positivity in the study sample who were not screened. Demographic variables and related risk factors were considered potential predictors, along with the screening site. The best-fit model was selected by AIC value. We utilized bootstrap validation to calculate an adjusted c-statistic.
Ethical Review
This study using retrospective de-identified health data was determined exempt from review under category IV by the University of Minnesota Institutional Review Board (IRB) (Study number 1605E87211), the Washington State IRB (Project number E-041516-H), and the Colorado Multiple IRB (Protocol number 18–0883); Thomas Jefferson University’s IRB approved this study under an expedited review (12 F.563). A CDC human subjects advisor determined that this project did not meet the definition of human subjects research under 45 CFR 46.102(d).
Results
Population Description
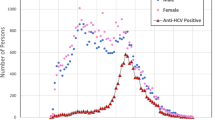
The participating sites submitted records for a combined total of 144,905 refugee arrivals. Among these, 153 were excluded due to missing or discrepant data. Among the remaining 144,752 records, 132,489 (91.5%) received a DME. Among those who received a DME, 64,703 (48.8%) were screened for hepatitis C antibodies (Table 1). The proportion of refugees screened by participating clinics ranges varied. Among clinics that performed at least 100 DMEs: 32% of clinics tested < 25% of arrivals for hepatitis C antibodies, and 48% of clinics tested ≥ 75% of arrivals for hepatitis C antibodies. Screening for hepatitis C antibodies increased with age; 34.6% of those 0–4-years were screened at the DME compared to 54.2% of those ≥ 65 years. Among refugees born from 1945 to 1965, 51% of those who received a DME after 2012 were tested for hepatitis C antibody, which is when CDC recommended universal screening for this cohort.
Hepatitis C Antibody Prevalence
Of the 64,703 refugees tested for hepatitis C antibodies at their DME, 1,030 (1.6%) had a positive hepatitis C antibody result (Table 2). Thirty-four (< 0.1%) had an indeterminate hepatitis C antibody result and were excluded from subsequent analyses. Among those ≥ 18 years at the time of screening, 912 (2.0%) tested positive.
Nationality
Among countries of origin with at least 10 individuals who tested positive for hepatitis C antibodies, the highest prevalence was observed among refugees from Burundi (5.4%), Moldova (3.8%), the Democratic Republic of Congo (3.2%), Burma (2.8%), and Ukraine (2.0%). Among those 18 years and older at the time of screening, the prevalence was highest among those from Burundi (8.5%), the Democratic Republic of Congo (4.8%), Moldova (4.7%), Burma (4.0%), and Ukraine (2.3%).
Sex
Among the 30,140 females screened for hepatitis C antibodies (excluding 11 with an indeterminate result), 428 (1.4%) tested positive. Among the 34,529 males screened for hepatitis C antibodies (excluding 23 with an indeterminate result), 602 (1.7%) tested positive. Among those 18 years and older, 380 (1.8%) females and 532 (2.2%) males tested positive.
Age
Among children younger than 5 years, 0.5% had hepatitis C antibodies detected, compared to 1.7% of adults 18–44 years, 3.2% of adults 45–64 years, and 4.3% of adults ≥ 65 years.
Hepatitis C Risk Factors
Compared to refugees from the Eastern Mediterranean region, refugees from the Americas had a 41% lower prevalence of hepatitis C antibodies (95% CI, 0.38–0.90), after adjusting for age and sex (Table 3). Refugees from Africa, South-East Asia, Western Pacific, and Europe had 3.6 (95% CI 3.0-4.4), 2.9 (2.5–3.5), 2.6 (1.2–5.9), and 2.4 (1.8–3.2) times higher adjusted prevalence of hepatitis C antibodies compared to refugees from the Eastern Mediterranean, respectively.
After adjustment for age and region of origin, males had a 25% increased prevalence of hepatitis C antibodies (95% CI 1.1–1.4) compared to females. Compared with children aged ≤ 5 years, refugees 18–44 (3.6 (95% CI 2.5–5.3)), 45–64 (7.1 (95% CI 4.8–10.5)), and ≥ 65 (9.1 (95% CI 5.8–14.1)) had a higher adjusted prevalence of hepatitis C antibodies. The adjusted prevalence of hepatitis C antibodies was not significantly higher for children 5–12 years or 13–17 years compared to children aged 5 years and younger.
Predictive Statistical Model
The sample was restricted to refugees ≥ 18 years. The selected model included age (Χ2 = 466.5, p < 0.001), sex (Χ2 = 196.5, p < 0.001), nationality (Χ2 = 9.7, p = 0.002), and the collaborating site (Χ2 = 29.7, p < 0.001) (adj. c-statistic = 0.75). Among adult refugees in this population who were not screened for hepatitis C antibodies during the DME, an estimated 1.2%, or 498 people, would have tested positive.
Discussion
Hepatitis C Screening
During 2010–2017, CDC DME guidelines only recommended hepatitis C screening for those with risk factors such as injection drug use or HIV infection and, starting in 2012, for those born from 1945 to 1965 [6]. The guidelines stated that it was reasonable to screen adults from countries with a hepatitis C prevalence ≥ 2%. Due to these recommendations, only 48.8% of refugees screened at the participating sites were tested for hepatitis C antibodies at their DME. At least 65.2% of those who received a DME would have been eligible for hepatitis C antibody screening under the 2020 CDC guidelines. An estimated 498 persons with hepatitis C antibody positivity were missed among unscreened adults. As a comparison, CDC recommends screening all refugees aged 13 to 64 years for HIV. Previous research indicates that 85.2% of adult refugees were screened for HIV among nine sites conducting the DME between 2014 and 2016 [12]. This emphasizes both the value added to screen all adult refugees for hepatitis C at the DME and the potential to increase screening based on other screening recommendations.
The proportion of those screened for hepatitis C antibodies during their DME varied significantly based on country of origin and age. Adults and those from higher incidence countries were screened at higher proportions. Males were also screened at a significantly higher proportion than females, which may have been due to risk-factor-based screening. Screening for hepatitis C is important for secondary prevention to reduce the long-term risk of cirrhosis and HCC [13].
Hepatitis C Antibody Prevalence
Adult refugees ≥ 18 years, in addition to males, had a significantly higher prevalence of hepatitis C antibodies. Refugees from Africa, Southeast Asia, Western Pacific, and Europe had a significantly higher prevalence of HCV antibodies than refugees from the Eastern Mediterranean. In contrast, refugees from the Americas had a significantly lower prevalence of hepatitis C antibodies than refugees from the Eastern Mediterranean. Adult refugees coming from Burundi, the Democratic Republic of Congo, Moldova, Burma, and Ukraine all had a crude hepatitis C antibody prevalence higher than 2%. A 2014 review of existing studies on global hepatitis C prevalence reported similar hepatitis C antibody prevalence for adults from the Democratic Republic of Congo (4.3%), Moldova (4.5%), Ukraine (3.6%), and Burma (1.7%) [14]. However, the estimated adult prevalence for Burundi was only 1-1.5%, which was lower than we observed in our study population [14]. Refugees may have a different risk of hepatitis C compared to others of the same nationality since many refugees have spent years living outside of their country of origin prior to resettlement in the United States, and they are often of different demographics and exposure risk than the general population [15, 16].
Hepatitis C Follow-up Care
The current DME guidelines recommend an HCV RNA polymerase chain reaction (PCR) test for refugees with a positive HCV antibody test [5]. People with a positive hepatitis C antibody test and negative HCV RNA test do not have hepatitis C. Positive hepatitis C antibodies and negative HCV RNA occur in the setting of resolved infections and false positive antibody screening tests [17]. CDC guidance recommends people with positive HCV RNA receive care management by a healthcare provider with expertise in chronic liver disease and hepatitis C [5]. Other recommendations for management of persons diagnosed with hepatitis C include vaccination for hepatitis A and hepatitis B, hepatitis C direct-acting antiviral (DAA) treatment, and ongoing management. CDC guidance also recommends that health education should be provided to patients with HCV infection, including prevention of transmission, signs and symptoms of cirrhosis, and HCC. The current American Association for the Study of Liver Diseases guidelines should be followed for treatment [18]. Linkage to care is an important next step for those with chronic hepatitis C. It is important to train healthcare providers on testing procedures and asking patients about hepatitis C risk factors [19]. Compared to private health insurance, Medicaid was associated with lower odds of being treated [20, 21]. However, treatment rates improved after Medicaid removed the specialist provider and advanced fibrosis restriction requirements for treatment. Without access to treatment and follow-up care, those with hepatitis C are at higher risk for end-stage liver disease [22]. Previous research indicates the cost-effectiveness of newer DAA treatments [23, 24].
Study Strengths and Limitations
This analysis is subject to several limitations. Refugees arriving to regions of the United States not included in this evaluation may have been underrepresented in these data. However, the refugee population included in this review represents nearly 28% of all newly arrived refugees in the United States during the study period. This study does not provide comprehensive global or regional estimations of hepatitis C prevalence in refugees because the study population was limited to countries of origin for newly arrived refugees in the United States. The prediction model is specific to this study sample and should not be applied beyond these collaborating sites. It also had only a fair c-statistic value suggesting unexplained variations in hepatitis C antibody prevalence which could affect the predicted estimate. We were unable to accurately estimate the prevalence of HCV infections, confirmed by HCV RNA, in this population due to differences in state reporting rules for HCV infections and the ability of each study site to obtain confirmatory testing results for this study. Many screening programs show a substantial decreased rate of return of patients who are identified as HCV antibody positive for confirmation of diagnosis by HCV RNA testing [25]. Reflex testing, or immediately performing an HCV RNA test on all with a positive HCV antibody using the same specimen, would improve state refugee health programs’ abilities to identify HCV infections. An improved linkage system between state refugee health databases and infectious disease surveillance systems would also allow us to better evaluate the prevalence of HCV infections confirmed by HCV RNA. Such analyses could further inform hepatitis C screening recommendations for refugees arriving in the United States by determining what percentage of refugee patients with positive hepatitis C antibodies ultimately test positive for HCV RNA and require linkage to care and treatment.
Refugee screening clinics employed differing screening protocols, which could affect prevalence estimates. However, there was no significant difference in prevalence between clinics that did and did not universally screen for hepatitis C antibodies. Additionally, it was not until 2012 that CDC recommended that all adults in the U.S. born from 1945 to 1965 receive universal screening for hepatitis C antibodies, which was midway through this study period. Even after this recommendation was in place, only 51% of refugees in this birth cohort were tested for hepatitis C antibodies at the DME. There was variation in the hepatitis C risk factor data that sites collected and included in this analysis. Future work should prioritize collecting standardized risk factor data based on CDC’s DME screening guidance including HIV in addition to longitudinal follow-up data beyond the DME. The updated CDC hepatitis C screening guidelines for refugees may ensure a more standard approach to hepatitis C screening in these populations. Finally, we could not determine the method of exposure to hepatitis C, which could be valuable for prevention.
Conclusions
The DME represents an opportunity to screen refugees for hepatitis C antibodies and complete a diagnostic HCV RNA test. DME updates in 2020 recommend universal hepatitis C screening for all new adult arrivals, all pregnant people during each pregnancy, all unaccompanied refugee minors, children with risk factors, and children born to mothers with hepatitis C. The findings demonstrate the highest prevalence of hepatitis C antibodies among adult refugees from Burundi, Moldova, Democratic Republic of Congo, Burma, and Ukraine. Future evaluations are needed to identify the prevalence of infection based on diagnostic testing and the rate of linkage to care and treatment among HCV-infected refugees.
References
Polaris Observatory HCV, Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modeling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. https://doi.org/10.1016/S2468-1253(16)30181-9.
Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of Hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–31. https://doi.org/10.1002/hep.30297.
Hepatitis C, Questions and Answers for Health Professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Published August 7, 2020. Accessed January 17, 2023.
Admissions & Arrivals. Refugee Processing Center. https://www.wrapsnet.org/admissions-and-arrivals/. Published 2021. Accessed August 13, 2021.
Refugee Health Guidance. Centers for Disease Control and Prevention. https://www.cdc.gov/immigrantrefugeehealth/guidelines/refugee-guidelines.html.Published March 16, 2021.
Screening for Viral Hepatitis During the Domestic Medical Examination of Newly Arrived Refugees. Centers for Disease Control and Prevention. https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/hepatitis-screening-guidelines.html. Published October 30, 2020. Accessed August 1, 2022.
Greenaway C, Ma AT, Kloda LA, et al. The seroprevalence of Hepatitis C antibodies in immigrants and Refugees from Intermediate and High Endemic Countries: a systematic review and Meta-analysis. PLoS ONE. 2015;10(11):e0141715. https://doi.org/10.1371/journal.pone.0141715.
Mixson-Hayden T, Lee D, Ganova-Raeva L, et al. Hepatitis B virus and hepatitis C virus infections in United States-bound refugees from Asia and Africa. Am J Trop Med Hyg. 2014;90(6):1014–20.
Shire AM, Sandhu DS, Kaiya JK et al. Viral hepatitis among Somali immigrants in Minnesota: association of hepatitis C with hepatocellular carcinoma. Mayo Clin Proc. 2012;87(1):17–24.
Crawshaw AF, Pareek M, Were J, et al. Infectious disease testing of UK-bound refugees: a population-based, cross-sectional study. BMC Med. 2018;16(1):143. https://doi.org/10.1186/s12916-018-1125-4.
Regional offices. World Health Organization. https://www.who.int/about/who-we-are/regional-offices. Accessed August 1, 2022.
Pezzi C, Lee D, Kumar G, et al. Health screenings administered during the domestic medical examination of refugees and other eligible immigrants in nine US states, 2014–2016: a cross-sectional analysis. PLoS Med. 2020;17(3):e1003065. https://doi.org/10.1371/journal.pmed.1003065.
Tafuri S, Prato R, Martinelli D, et al. Prevalence of Hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infect Dis. 2010;10(1). https://doi.org/10.1186/1471-2334-10-213.
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):45–S57.
Uddin G, Shoeb S, Solaiman S, et al. Prevalence of chronic viral hepatitis in people of south asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. J Viral Hepat. 2010;17(5):327–35. https://doi.org/10.1111/j.1365-2893.2009.01240.x.
Global progress report on HIV, viral hepatitis and sexually transmitted infections., 2021 Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO.
Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol. 2017;89:1–4. https://doi.org/10.1016/j.jcv.2017.01.007.
Guidance HCV. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. https://www.hcvguidelines.org. Published 2020. Accessed August 1, 2022.
Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep. 2020;10(2):1–17. https://doi.org/10.15585/mmwr.rr6902a1.
Bourgi K, Brar I, Baker-Genaw K. Health disparities in hepatitis C screening and linkage to care at an integrated health system in southeast Michigan. PLoS ONE. 2016;11(8):e0161241. https://doi.org/10.1371/journal.pone.0161241.
Nephew LD, Wang Y, Mohamed K, et al. Removal of medicaid restrictions were associated with increased hepatitis C virus treatment rates, but disparities persist. J Viral Hepat. 2022;29(5):366–74. https://doi.org/10.1111/jvh.13661.
Di Marco L, La Mantia C, Di Marco V, Hepatitis C. Standard of Treatment and what to do for global elimination. Viruses. 2022;14(3):505. https://doi.org/10.3390/v14030505.
Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61(2):157–68. https://doi.org/10.1093/cid/civ220.
Mattingly TJII, Slejko JF, Onukwugha E, Perfetto EM, Kottilil S, Mullins CD. Value in hepatitis C virus treatment: a patient-centered cost-effectiveness analysis. PharmacoEconomics. 2020;38(2):233–42. https://doi.org/10.1007/s40273-019-00864-8.
Mera J, Vellozzi C, Hariri S, et al. Identification and clinical management of persons with chronic Hepatitis C virus infection — Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep. 2016;65(18):461–6. https://doi.org/10.15585/mmwr.mm6518a2.
Acknowledgements
This project was supported by the CDC Centers for Excellence in Newcomer Health grant 5 NU50CK000459. CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Urban, K., Payton, C., Mamo, B. et al. Hepatitis C Screening and Antibody Prevalence Among Newly Arrived Refugees to the United States, 2010–2017. J Immigrant Minority Health 25, 1323–1330 (2023). https://doi.org/10.1007/s10903-023-01471-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10903-023-01471-8