Abstract
Gambling Disorder (GD) is a condition constituting a public health concern, with a burden of harm which is much greater than that of drug addiction. Patients with GD are generally reluctant to pharmacologic treatment and seem to prefer nonpharmacological interventions. Therefore, this proof-of-concept study aimed to investigate the feasibility of continuous Theta Burst Stimulation (cTBS) on the pre-SMA in six patients (5 males, 1 female), aged 30–64 years, with a DSM-5 diagnosis of Gambling Disorder and no comorbid mood disorders. Participants received over 10 sessions of Continuous TBS (cTBS) over pre-SMA bilaterally and have been evaluated using rating scales, including the PG-YBOCS and the CGI, before treatment (T0), at day 10 of treatment (T1) and at day 30 after treatment (T2); cTBS intervention was safe and without side effects. Since the design of our study does not allow us to draw conclusions on the effectiveness of the intervention with respect to the improvement of the functioning of the subject with GD, a more in-depth study, including a sham condition, neurocognitive measures of disinhibition and decision making, and collecting follow-up data on the sustained effect of TBS over a longer period is ongoing.
Similar content being viewed by others
Introduction
Gambling Disorder (GD) has been recently re-classified in the DSM-5 under the “substance-related and addictive disorders”, considering its genetic, endophenotypic, and phenotypic resemblances to substance dependence. GD also displays growing epidemiology: recent studies report adult problem gambling prevalence rates in the past 12 months ranging from 0.5 to 3.0%, with three to four times as many people reporting subclinical problems and harm (Abbott, 2020). Gambling-related burden of harm has been shown in two studies to be approximately two-thirds to three-quarters that of major depressive disorder and alcohol misuse and dependence and three times that of drug dependence. Harm is predominantly due to financial impacts, damage to health and relationships, psychological distress and adverse impacts on education and work (Abbott, 2020). During the Covid-19 pandemic, it has been reported that the lockdown and social distancing may have exerted an impact even on gambling behavior, not only by increasing gambling behavior in those affected by this disorder but even contributing to the occurrence of new cases, with a consistent proportion of business owners and unemployed individuals reported problem gambling during the lockdown period (Salerno & Pallanti, 2021). After over twenty years of neurobiological research on GD neurocircuitry, studies have found in people with GD alterations in decision-making processes (Pettorruso et al., 2019) and diminished control over behaviors, suggestive of faulty inhibitory control mechanisms (Verdejo-Garcia et al., 2015). An interesting metanalysis conducted by Ioannidis and colleagues showed heightened impulsivity across a range of cognitive domains in GD (Ioannidis et al., 2019). Neuroimaging studies have shown relative glucose metabolic rates (rGMR) in the orbitofrontal cortex and medial frontal cortex that were significantly increased at baseline in GD patients compared to normal controls (Hollander et al., 2008), a decrement of the rGMR in the ventral parts of the striatum and thalamus, and an increment of the rGMR in the dorsal parts as compared with the controls (Pallanti et al., 2010). In addition, a serotonergic dysfunction in GD has also been reported (Pallanti, Bernardi, Allen & Hollander 2010), which was similar to that reported in people with Alcohol Use Disorder (Clark et al. 2019), as well as a peripheral noradrenergic dysfunction that could be consistent with attenuated cortico-frontal noradrenergic function as shown in positron emission tomography (PET) studies of GD (Pallanti, Bernardi, Allen, Chaplin et al. 2010). On the basis of this evidence, it also emerges that the prefrontal, orbital and ventromedial regions may be a possible target for treatment in GD as they contribute to emotional and affective regulation and cognitive control (Moccia L. et al., 2017). Unfortunately, few people seek treatment for GD, and of those with GD less than 15% receive treatment (Slutske, 2006), and almost none with less severe problems do (Petry, 2005). Although no treatment is clinically validated for GD, Cognitive Behavioral (CB) interventions have the greatest evidence of efficacy (Petry et al. 2017). From a pharmacological point of view, to date no pharmacological therapy has a formal indication or has been approved for the treatment of GD. Of the placebo-controlled studies, results showed that opioid antagonists and mood stabilizers may be helpful (Goslar et al. 2019; Potenza et al. 2019). In particular, lithium treatment may reduce cognitive dysfunction and symptoms in GD patients (Goslar et al., 2019) and topiramate may reduce clinically important impulsivity in GD (Berlin et al., 2013). On the other hand, by decreasing dopamine neurotransmission in the nucleus accumbens and the motivational neurocircuitry, opioid antagonists reduce gambling excitement and craving (Victorri-Vigneau et al., 2018, Grant et al. 2006). Consequently to the limited effect of the pharmacological treatment in GD more recently, circuitries-targeted non-invasive brain stimulation (NIBS) techniques have grasped the interest showing the possibility to modulate selectively brain regions associated with dysfunctions involved in the cycle of Gambling Disorder. Since these allow re-modulation of aberrant brain networks by promoting or inhibiting neural activity in specific regions and related networks, there has been a growing interest towards NIBS for impulsive-compulsive spectrum disorders in the last 15 years and, more recently, for substance use disorders (SUDs) (Stein et al., 2018; Ekhtiari et al., 2019). Repetitive transcranial magnetic stimulation (rTMS) emerged as the most employed technique to modulate both decision-making and reward networks. For what concerns gambling, rTMS studies mainly focused on the dorsolateral prefrontal cortex (DLPFC) as target, with most protocols activating the left DLPFC (Sheffer et al., 2013; Gay et al., 2017; Pettorruso et al., 2019; 2020). Recently, also Theta Burst Stimulation (TBS) has been investigated to modulate gambling reinforcement, delay discounting and stroop interference in GD (Zack et al., 2016). By contrast, the pre-supplementary motor area (pre-SMA) has been scarcely investigated in rTMS trials, whereas it may represent a core region as target for response inhibition since previous studies reported its role in effective reduction of risky decisions and improvement of inhibitory control (Obeso 2017; Tosun 2017). In consideration of what has been said so far, the aim of the present study is to investigate the effect of continuous Theta Burst Stimulation (cTBS) on the pre-SMA as preliminary part of a broader investigation aiming to study the mechanism of response inhibition in GD, which is ongoing by the same group of researchers. The design of this study doesn’t allow to consider it as a clinical trial but rather as a proof of concept, which can provide interesting insights for subsequent research.
Participants
Six patients with a diagnosis of GD (5 males, 1 female; average age was 45.7, range 30–64) were consecutively admitted at the INS, that it is a well-known Research Private Center for its involvement in the therapy on GD and research in TMS, to receive 10 sessions of Continuous TBS (cTBS) over the pre-SMA bilaterally. Participants were aged 18 and over, had a GD diagnosis according to criteria of the DSM-5 (APA, 2013), a history of illness of at least 1 year and a PG-YBOCS score of 16 or above. Exclusion criteria were no actual comorbidity for mood disorders, the presence of a risk of seizure or epilepsy, implanted devices, metal in the brain, pregnancy (investigated by the TMS Safety Screening Questionnaire) and neurological disorders.
The study was design as open-label and was approved by the local ethics committee and, after complete description of the study to the subjects, written informed consent was obtained.
Procedures
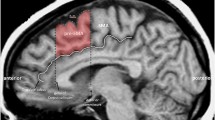
All procedures used in this study were reviewed and approved through the University human research IRB. cTBS was administered with the MagVenture MagPro R30 stimulator with add-on Theta Burst option (MagVenture INC.) using a Cool D-B80 figure-of-eight coil. TBS consists of bursts of 3 pulses separated by 20ms (i.e., 50 Hz), with each triplet being repeated every 200 ms (i.e., 5 Hz). Stimulus intensities were set at 80% of RMT. 2 trains of 600 pulses each separated by 1 min (a total of 1200 pulses) were used. cTBS was applied according to established safety guidelines. The bilateral pre-SMA was targeted using individual MRI and a neuronavigation system. Resting motor threshold (RMT) was defined as the minimum magnetic flux needed to elicit a threshold EMG response (50 mV in peak-to-peak amplitude) in a resting target muscle (abductor pollicis brevis or APB) in 5/10 trials using single-pulse TMS administered to the contralateral primary motor area.
Baseline and Follow-up Assessments
Baseline assessments were performed before the first cTBS session. At baseline subjects underwent a psychiatric interview conducted by senior psychiatrists, followed by a comprehensive clinical interview. Diagnosis was performed using the DSM-5 criteria (APA 2013), the current severity of the subject’s GD symptoms was measured by the The Pathological Gambling version of the Yale-Brown Obsessive-Compulsive Scale (PG-YBOCS). Other related conditions, such as anxiety and depression, were also measured using the Hamilton Anxiety Scale (HAM-A), and the Hamilton Depression Scale (HAM-D). Other assessment tools included the Gambling Urges Questionnaire (GUQ), the Barratt Impulsiveness Scale (BIS-11), Sheehan Disability Scale (SDS), the Clinical Global Impression Scale (CGI) and the Fagerstrom Test for Nicotine Dependence (FTND). Previous and current medications were recorded as well. The outcome measures were repeated at day 10 of treatment (T1), at day 30 after treatment (T2). Additional baseline data have been obtained by interview and review of hospital records. Safety and tolerability were monitored by assessing each week adverse events and vital signs.
Outcome Measures
The primary outcome measure was the reduction of disease severity according to PG-YBOCS (score range: 0–40). The outcome measurements were performed at baseline (T0), after 10 cTBS stimulations (T1) and at 30 days after the end of treatment (T2).
Our institutional review board IRB approved the study in accordance with the Helsinki Declarations of 1975. After a complete description of the study to the subjects, written informed consent was obtained.
Statistical Analysis
A Friedman test was run to determine if there were differences in PG-YBOCS and the other rating scales during treatment. As multiple comparisons increase the risk of a Type I error, pairwise comparisons were performed (SPSS Statistics) with a Bonferroni correction for multiple comparisons. Statistical significance was set at p > .05, two-sided.
Results
At the baseline, the average PG-YBOCS score was 21.5 (range 18–26), average GUQ score was 29.8 (range 20–36), average BIS-11 score was 69 (range 59–76), average HAM-A was 9.2 (range 1–13), average HAM-D was 5 (range 2–7), average SDT score was 18.8 (range 12–30), average FTND score was 3 (range 0–10) and average CGI was 3.8 (range 3–5). PG-YBOCS score was statistically significantly different at the different time points during the treatment intervention, χ2(2) = 10.174, p = .006 (2-sided). Post hoc analysis revealed statistically significant differences in PG-YBOCS scores between baseline and mid- (p = .03) and post-treatment (p = .002) but not between mid- and post-treatment (p = .386). A statistically significant difference also has been found in CGI scores during intervention χ2(2) = 11.474, p = .003 (2-sided). Post hoc analysis revealed statistically significant differences in CGI scores between baseline and mid- (p = .006) and post-treatment (p = .014) but not between mid- and post-treatment (p = .773). Conversely, no statistically significant differences have been found by comparing the other rating scales scores at the different time points, as shown in Table 1.
Discussion
The main result of this proof-of-concept trial of cTBS over the pre-SMA in six GD patients is the severity decrease of GD as shown by the significant reduction of the PG-YBOCS score during the intervention (p = .006), along with that of CGI (p = .003), indicating an amelioration of the whole clinical picture. Regarding the reduction in GD symptomatology, it is to highlight that the PG-YBOCS scores declined to a non-clinical level for all six patients. To the best of our knowledge, compared to other treatment studies, the percentage of successful treatment appears to be high, and this could be partly explained by the placebo effect due to the open design of the study. Indeed, mediators such as the therapeutic alliance established by regular contacts between patients and therapists, patients` expectations to benefit from treatment, learning processes associated with drug stimuli (classical conditioning), elevated levels of motivation to change problematic behavior, or the natural recovery from gambling are all aspects which are extensively discussed in the literature (e.g., Finniss et al., 2010; Grant and Chamberlain 2017; Prochaska et al. 1992; Schedlowski et al. 2015; Slutske 2006). Moreover, symptom improvement may not always lead to an increase in quality of life, and this aspect will be evaluated with a research study with a different design. A six months of follow-up period after treatment cessation with no additional sessions may provide some insights on the treatment efficacy.
A relevant distinction of this study is the lack of dropouts (which are quite common in GD population undergoing treatments for the disorder) and a great tolerability of the TBS treatment, as no adverse effects have been reported.
The main limitations of this study consist in its open design, the small sample, the lack of a control arm, the short duration of the treatment protocol and the low number of pulses. Since neurocognitive measures of disinhibition and decision making have been positively associated with the severity of problem gambling and may predict relapse of disordered gambling (Goudriaan et al. 2008), future investigation might consider the use of multidimensional measures of changes rather than global ones. Since symptom reduction does not equate to improved functioning of the subject and his or her quality of life, further research is needed to see if our study is associated with any changes in inhibitory control and decision-making abilities.
Considering all these limitations, our proof-of-concept study provides interesting results interesting results that are worth addressing with further research that is currently underway.
Conclusions
Gambling Disorder is a condition constituting a public health concern, as it is associated with detrimental consequences for affected individuals and has very high social costs. The design of our study does not allow us to draw conclusions on the effectiveness of the intervention with respect to the improvement of the functioning of the subject with GD, but this objective was not set either. However, it showed that cTBS is a safe treatment without side effects, which in our sample of subjects was associated with a reduction in the scores of the scales that measure the severity of GD down to below the cut-off. It is therefore worth doing a more in-depth study, that will benefit from including a sham condition as a comparator condition, neurocognitive measures of disinhibition and decision making, and collect follow-up data on the sustained effect of TBS over a longer period.
References
Abbott, M. W. (2020). The changing epidemiology of gambling disorder and gambling-related harm: public health implications. Public health, 184, 41–45. https://doi.org/10.1016/j.puhe.2020.04.003
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.)
Berlin, H. A., Braun, A., Simeon, D., Koran, L. M., Potenza, M. N., McElroy, S. L. … Hollander, E. (2013 Mar). A double-blind, placebo-controlled trial of topiramate for pathological gambling. World J Biol Psychiatry, 14(2), 121–128
Ekhtiari, H., Tavakoli, H., Addolorato, G., Baeken, C., Bonci, A., Campanella, S. … Hanlon, S. C., C. A (2019). Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neuroscience and biobehavioral reviews, 104, 118–140. https://doi.org/10.1016/j.neubiorev.2019.06.007
Finniss, D. G., Kaptchuk, T. J., Miller, F., & Benedetti, F. (2010). Biological, clinical, and ethical advances of placebo effects. Lancet, 375, 686–695. https://doi.org/10.1016/S0140-6736(09)61706-2
Hollander, E., Buchsbaum, M. S., Haznedar, M. M., Berenguer, J., Berlin, H. A., Chaplin, W. … Pallanti, S. (2008). FDG-PET study in pathological gamblers. 1. Lithium increases orbitofrontal, dorsolateral and cingulate metabolism. Neuropsychobiology, 58(1), 37–47. https://doi.org/10.1159/000154478
Gay, A., Boutet, C., Sigaud, T., Kamgoue, A., Sevos, J., Brunelin, J., & Massoubre, C. (2017 Mar). A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. Eur Psychiatry, 41, 68–74
Grant, J. E., Kim, S. W., & Hartman, B. K. (2008). A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. The Journal of clinical psychiatry, 69(5), 783–789. https://doi.org/10.4088/jcp.v69n0511
Grant, J. E., Potenza, M. N., Hollander, E., Cunningham-Williams, R., Nurminen, T., Smits, G., & Kallio, A. (2006). Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. The American journal of psychiatry, 163(2), 303–312. https://doi.org/10.1176/appi.ajp.163.2.303
Goudriaan, A. E., Oosterlaan, J., De Beurs, E., & Van Den Brink, W. (2008). The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision-making in the prediction of relapse in pathological gamblers. Psychological medicine, 38(1), 41?50. https://doi.org/10.1017/S0033291707000694.
Obeso, I., Wilkinson, L., Teo, J. T., Talelli, P., Rothwell, J. C., & Jahanshahi, M. (2017 Sep-Oct). Theta burst magnetic stimulation over the pre-supplementary motor area improves motor inhibition. Brain Stimul, 10(5), 944–951
Pallanti, S., Haznedar, M. M., Hollander, E., Licalzi, E. M., Bernardi, S., Newmark, R., & Buchsbaum, M. S. (2010). Basal Ganglia activity in pathological gambling: a fluorodeoxyglucose-positron emission tomography study. Neuropsychobiology, 62(2), 132–138
Pallanti, S., Bernardi, S., Allen, A., & Hollander, E. (2010). Serotonin function in pathological gambling: blunted growth hormone response to sumatriptan. J Psychopharmacol, 24(12), 1802–1809
Clark, L., Boileau, I., & Zack, M. (2019 May). Neuroimaging of reward mechanisms in Gambling disorder: an integrative review. Mol Psychiatry, 24(5), 674–693. doi: https://doi.org/10.1038/s41380-018-0230-2
Pallanti, S., Bernardi, S., Allen, A., Chaplin, W., Watner, D., DeCaria, C. M., & Hollander, E. (2010 Jun). Noradrenergic function in pathological gambling: blunted growth hormone response to clonidine. J Psychopharmacol, 24(6), 847–853
Moccia, L., Pettorruso, M., De Crescenzo, F., De Risio, L., di Nuzzo, L., Martinotti, G. … Di Nicola, M. Neural correlates of cognitive control in gambling disorder: a systematic review of fMRI studies.Neurosci Biobehav Rev. 2017Jul; 78:104–116. doi: https://doi.org/10.1016/j.neubiorev.2017.04.025
Petry, N. M. (2005). Pathological gambling: Etiology, comorbidity, and treatment. Washington, DC: American Psychological Association
Petry, N. M., Ginley, M. K., & Rash, C. J. (2017 Dec). A systematic review of treatments for problem gambling. Psychol Addict Behav, 31(8), 951–961. doi: https://doi.org/10.1037/adb0000290. Epub 2017 Jun 22. PMID: 28639817; PMCID: PMC5714688
Pettorruso, M., Di Giuda, D., Martinotti, G., Cocciolillo, F., De Risio, L., Montemitro, C., Camardese, G., et al. (2019 Jun). Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: a SPECT case study. Addict Behav, 93, 246–249
Pettorruso, M., Martinotti, G., Cocciolillo, F., De Risio, L., Cinquino, A., Di Nicola, M., Camardese, G., et al. (2019 Sep). Striatal presynaptic dopaminergic dysfunction in gambling disorder: A 123 I-FP-CIT SPECT study. Addict Biol, 24(5), 1077–1086
Pettorruso, M., Martinotti, G., Montemitro, C., De Risio, L., Spagnolo, P. A., Gallimberti, L., Fanella, F., et al. (2020). Multiple Sessions of High-Frequency Repetitive Transcranial Magnetic Stimulation as a Potential Treatment for Gambling Addiction: A 3-Month, Feasibility Study. Eur Addict Res, 26(1), 52–56
Prochaska, J. O., DiClemente, C. C., & Norcross, J. C. (1992 Sep;47(9):1102-14). In search of how people change. Applications to addictive behaviors. Am Psychol. doi: https://doi.org/10.1037//0003-066x.47.9.1102. PMID: 1329589
Salerno, L., & Pallanti, S. COVID-19 Related Distress in Gambling Disorder.Front Psychiatry. 2021 Feb25; 12:620661
Schedlowski, M., Enck, P., Rief, W., & Bingel, U. (2015 Jul;67(3):697–730). Neuro-Bio-Behavioral Mechanisms of Placebo and Nocebo Responses: Implications for Clinical Trials and Clinical Practice. Pharmacol Rev. doi: https://doi.org/10.1124/pr.114.009423. PMID: 26126649
Sheffer, C. E., Mennemeier, M., Landes, R. D., Bickel, W. K., Brackman, S., Dornhoffer, J. … Brown, G. (2013 Aug). Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J Subst Abuse Treat, 45(2), 206–214
Slutske, W. S. (2006). Natural recovery and treatment-seeking in pathological gambling: results of two US national surveys. American Journal of Psychiatry, 163(2), 297–302
Stein, E. R., Gibson, B. C., Votaw, V. R., Wilson, A. D., Clark, V. P., & Witkiewitz, K. (2019). Non-invasive brain stimulation in substance use disorders: implications for dissemination to clinical settings. Curr Opin Psychol, 30, 6–10
Tosun, T., Berkay, D., Sack, A. T., Çakmak, Y., & Balcı, F. (2017 Aug). Inhibition of Pre-Supplementary Motor Area by Continuous Theta Burst Stimulation Leads to More Cautious Decision-making and More Efficient Sensory Evidence Integration. J Cogn Neurosci, 29(8), 1433–1444
Verdejo-Garcia, A., & Manning, V. (2015). Executive Functioning in Gambling Disorder: Cognitive Profiles and Associations with Clinical Outcomes. Curr. Addict. Rep, 2, 214–219
Ioannidis, K., Hook, R., Wickham, K., Grant, J. E., & Chamberlain, S. R. (2019). Impulsivity in Gambling Disorder and problem gambling: a meta-analysis. Neuropsychopharmacolog: official publication of the American College of Neuropsychopharmacology, 44(8), 1354–1361
Victorri-Vigneau, C., Spiers, A., Caillet, P., et al. (2018). Opioid Antagonists for Pharmacological Treatment of Gambling Disorder: Are they Relevant? Curr Neuropharmacol, 16(10), 1418–1432
Zack, M., Cho, S. S., Parlee, J., Jacobs, M., Li, C., Boileau, I., & Strafella, A. (2016). Effects of High Frequency Repeated Transcranial Magnetic Stimulation and Continuous Theta Burst Stimulation on Gambling Reinforcement, Delay Discounting, and Stroop Interference in Men with Pathological Gambling. Brain Stimul. Nov-Dec;9(6):867–875
Goslar, M., Leibetseder, M., Muench, H. M., Hofmann, S. G., & Laireiter, A. R. (2019). Pharmacological treatments for disordered gambling: A meta analysis. Journal of Gambling Studies, 35(2), 415–445. https://doi.org/10.1007/s10899-018-09815-y
Potenza, M.N., Balodis, I.M., Derevensky, J. et al. Gambling disorder. Nat Rev Dis Primers 5, 51 (2019). https://doi.org/10.1038/s41572-019-0099-7
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award ***********. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Author information
Authors and Affiliations
Contributions
SP developed the idea, and all authors made a substantial contribution to the development and writing of this article. LS acting as corresponding author, had the final responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Conflict of Interest
We declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salerno, L., Grassi, E., Makris, N. et al. “A Theta Burst Stimulation on Pre-SMA: Proof-of-Concept of Transcranial Magnetic Stimulation in Gambling Disorder”. J Gambl Stud 38, 1529–1537 (2022). https://doi.org/10.1007/s10899-022-10129-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10899-022-10129-3