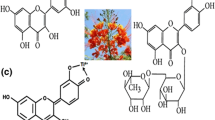
Abstract
In this work, we focused on extracting the anthocyanin dye in acetone, butanol, ethanol, and water solvents from Delonix regia flowers by a simple maceration extraction process. The identification of functional group analysis, vibrational studies, energy transfer mechanisms, optoelectronic properties, photostability studies, FRET-assisted potential light emissions and photometric properties of the anthocyanin dyes are successively investigated. FTIR spectroscopy and vibrational studies have confirmed the existence of polyphenolic groups in 2-phenyl chromenylium (anthocyanin) dyes. The optoelectronic results show the least direct bandgap (2.04 eV), indirect bandgap (1.55 eV), Urbach energy (0.380 eV), high refractive index (1.20), dielectric constant (2.794), and high optical conductivity (1.954 × 103 S/m) for the anthocyanin dye extracted found in water solvent. The photoluminescence properties such as Stoke’s shift, high quantum yield, and lifetime results show that anthocyanin dyes are promising candidates for red-LEDs and optical materials. The absorption and emission spectra of the anthocyanin dyes follow the mirror image rule and the Franck-Condon factor exists between vibrational energy levels corresponding to all the electronic transitions. The excellent correspondence between the absorption and emission spectra reinforces that the anthocyanins are efficient (46%) FRET probes. Further, photometric properties such as CIE, CRI, CCT and colour purity results of anthocyanins in all studied solvents revealed that this material exhibits orange to red shades (x = 0.48 → 0.54 and y = 0.36 →0.45) and is well suitable for have great potential in the manufacturing of Organic-LEDs and other optoelectronic device applications.






















Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Hussaan M, Iqbal N, Adeel S, Azeem M, Tariq Javed M, Raza A (2017) Microwave-assisted enhancement of milkweed (Calotropis procera L.) leaves as an eco-friendly source of natural colorants for textile. Environ Sci Pollut Res 24:5089–5094. https://doi.org/10.1039/D1RA01043C
Haripadmam PC, Thanuja TH, Varsha GS, Beryl CD, Aji A (2021) Nonlinear optical absorption in PVA films doped by the novel natural dye extract from C. redflash leaves. Opt Mater 112:110804. https://doi.org/10.1016/j.optmat.2021.110804
Bouchouit K, Derkowska B, Migalska-Zalas A, Abed S, Benali-Cherif N, Sahraoui B (2010) Nonlinear optical properties of selected natural pigments extracted from spinach: carotenoids. Dyes Pigm 86(2):161–165. https://doi.org/10.1016/j.dyepig.2009.12.013
Sugimoto A, Ochi H, Fujimura S, Yoshida A, Miyadera T, Tsuchida M (2004) Flexible OLED displays using plastic substrates. IEEE J Sel Top Quantum Electron 10(1):107–114. https://doi.org/10.1109/JSTQE.2004.824112
Fleetham T, Ecton J, Wang Z, Bakken N, Li J (2013) Single-doped white organic light‐emitting device with an external quantum efficiency over 20%. Adv Mater 25(18):2573–2576. https://doi.org/10.1002/adma.201204602
Reineke S, Lindner F, Schwartz G, Seidler N, Walzer K, Lüssem B, Leo K (2009) White organic light-emitting diodes with fluorescent tube efficiency. Nature 459(7244):234–238. https://doi.org/10.1038/nature08003
Jang E, Jun S, Jang H, Lim J, Kim B, Kim Y (2010) White-light‐emitting diodes with quantum dot colour converters for display backlights. Adv Mater 22(28):3076–3080. https://doi.org/10.1002/adma.201000525
Wang J, Lin W, Li W (2013) Three-channel fluorescent sensing via organic white light-emitting dyes for detection of hydrogen sulfide in living cells. Biomaterials 34(30):7429–7436. https://doi.org/10.1016/j.biomaterials.2013.06.013
Grimsdale AC, Leok Chan K, Martin RE, Jokisz PG, Holmes AB (2009) Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem Rev 109(3):897–1091. https://doi.org/10.1021/cr000013v
Miao Y, Yin M (2022) Recent progress on organic light-emitting diodes with phosphorescent ultra-thin (< 1nm) light-emitting layers. Iscience 25:103804. https://doi.org/10.1016/j.isci2022.103804
Yahya M, Bouziani A, Ocak C, Seferoğlu Z, Sillanpää M (2021) Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): recent developments, new trends, and future perceptions. Dyes Pigm 192:109227. https://doi.org/10.1016/j.dyepig.2021.109227
Goel A, Kumar V, Singh SP, Sharma A, Prakash S, Singh C, Anand RS (2012) Non-aggregating solvatochromic bipolar benzo [f] quinolines and benzo [a] acridines for organic electronics. J Mater Chem 22(30):14880–14888. https://doi.org/10.1039/c2jm31052j
Justin Thomas KR, Venkateswararao A, Joseph V, Kumar S, Jou JH (2019) Polarity tuning of fluorene derivatives by chromophores to achieve efficient blue electroluminescent materials org. Electron 64:266–273. https://doi.org/10.1016/J.ORGEL.2018.10.029
Sun W, Zhou N, Xiao Y, Wang S, Li X (2017) A Novel Spiro [acridine-9, 9′‐fluorene] derivatives containing phenanthroimidazole moiety for deep‐blue OLED application. Chem Asian J 12(23):3069–3076. https://doi.org/10.1002/asia.201701292
Jou JH, Kumar S, Jou YC (2013) Disruptive characteristics and lifetime issues of OLEDs. Organic Light-Emitting Diodes (OLEDs). Woodhead Publishing, United States. https://doi.org/10.1533/9780857098948.2.410
Singh V, Mishra AK (2016) White light emission from an aqueous vegetable cocktail: application towards pH sensing. Dyes Pigm 125:362–366. https://doi.org/10.1016/j.dyepig.2015.10.017
Park YI, Postupna O, Zhugayevych A et al (2015) A new pH-sensitive fluorescent and white light emissive material through controlled intermolecular charge transfer. Chem Sci 6(1):789–797. https://doi.org/10.1039/C4SC01911C
Singh V, Mishra AK (2015) White light emission from vegetable extracts. Sci Rep 5(1):11118. https://doi.org/10.1038/srep11118
Maiti DK, Bhattacharjee R, Datta A, Banerjee A (2013) Modulation of fluorescence resonance energy transfer efficiency for white light emission from a series of stilbene-perylene based donor–acceptor pair. J Phys Chem C 117(44):23178–23189. https://doi.org/10.1021/jp409042p
Singh V, Mishra AK (2016) White light emission from a mixture of pomegranate extract and carbon nanoparticles obtained from the extract. J Mater Chem C 4(15):3131–3137. https://doi.org/10.1039/C6TC00480F
Benelhadj K, Muzuzu et al (2014) White emitters by tuning the excited-state intramolecular proton‐transfer fluorescence emission in 2‐(2′‐hydroxybenzofuran) benzoxazole dyes. Chem – Eur J 20(40):12843–12857. https://doi.org/10.1002/chem.201402717
Maity A, Ali F, Agarwalla H, Anothumakkool B, Das A (2015) Tuning of multiple luminescence outputs and white-light emission from a single gelator molecule through an ESIPT coupled AIEE process. Chem Commun 51(11):2130–2133. https://doi.org/10.1039/C4CC09211B
Molla MR, Ghosh S (2012) Hydrogen-bonding‐mediated J‐aggregation and white‐light emission from a remarkably simple, single‐component, naphthalenediimide chromophore. Chem – Eur J 18(5):1290–1294. https://doi.org/10.1002/chem.201103600
Liu Y, Nishiura M, Wang Y, Hou Z (2006) π-Conjugated aromatic enynes as a single-emitting component for white electroluminescence. J Am Chem Soc 128(17):5592–5593. https://doi.org/10.1021/ja058188f
Zhang Y, Xie C, Su H, Liu J (2011) Employing heavy metal-free colloidal quantum dots in solution-processed white light-emitting diodes. Nano Lett 11(2):329–332. https://doi.org/10.1021/nl1021442
Liang R, Yan D et al (2014) Quantum dots-based flexible films and their application as the phosphor in white light-emitting diodes. Chem Mater 26(8):2595–2600. https://doi.org/10.1021/cm404218y
Chen KJ, Han HV, Chen HC et al (2014) White light-emitting diodes with enhanced CCT uniformity and luminous flux using ZrO2 nanoparticles. Nanoscale 6(10):5378–5383. https://doi.org/10.1039/C3NR06894C
Nicolai HT, Hof A, Blom PW (2012) Device physics of white polymer light-emitting diodes. Adv Funct Mater 22(10):2040–2047. https://doi.org/10.1002/adfm.201102699
Deng C, Jiang P, Shen X, Ling J, Hogen-Esch TE (2014) White light emission of multi-chromophore photoluminescent nanoparticles using polyacrylate scaffold copolymers with pendent polyfluorene groups. Polym Chem 5(17):5109–5115. https://doi.org/10.1039/C4PY00595C
Sun CY, Wang XL et al (2013) Efficient and tunable white-light emission of metal–organic frameworks by iridium-complex encapsulation. Nat Commun 4(1):2717. https://doi.org/10.1038/ncomms3717
Tang Q, Liu S, Liu Y, He D et al (2014) Colour tuning and white light emission via in situ doping of luminescent lanthanide metal–organic frameworks. Inorg Chem 53(1):289–293. https://doi.org/10.1021/ic402228g
Yang QY, Wu K, Jiang J et al (2014) Pure white-light and yellow-to-blue emission tuning in single crystals of Dy (III) metal–organic frameworks. Chem Commun 50(57):7702–7704. https://doi.org/10.1039/C4CC01763C
Sessolo M, Tordera D, Bolink HJ (2013) Ionic iridium complex and conjugated polymer used to solution-process a bilayer white light-emitting diode. ACS Appl Mater Interfaces 5(3):630–634. https://doi.org/10.1021/am302033k
Ramya AR, Varughese S, Reddy ML (2014) Tunable white-light emission from mixed lanthanide (Eu 3+, gd 3+, tb 3+) coordination polymers derived from 4-(dipyridin-2-yl) aminobenzoate. Dalton Trans 43(28):10940–10946. https://doi.org/10.1039/C4DT00871E
Xu Q, Yang W, Wen Y, Liu S, Liu Z, Ong WJ, Li N (2019) Hydrochromic full-colour MXene quantum dots through hydrogen bonding toward ultrahigh-efficiency white light-emitting diodes. Appl Mater Today 16:90–101. https://doi.org/10.1016/j.apmt.2019.05.001
Kumbhakar P, Biswas S et al (2019) Tailoring of structural and photoluminescence emissions by Mn and Cu co-doping in 2D nanostructures of ZnS for the visualization of latent fingerprints and generation of white light. Nanoscale 11(4):2017–2026. https://doi.org/10.1039/C8NR09074B
Poddubny AN, Rodina AV (2016) Nonradiative and radiative Förster energy transfer between quantum dots. J Exp Theor Phys 122:531–538. https://doi.org/10.1134/S1063776116030092
Yuan LIN, Lin W, Zheng K, Zhu S (2013) FRET-based small-molecule fluorescent probes: rational design and bioimaging applications. Acc Chem Res 46(7):1462–1473. https://doi.org/10.1021/ar300273v
Forster T (1948) Intermolecular energy transfer and fluorescence. Ann Phys, Leipzig Germany
Ledwon P (2019) Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications. Org Electron 75:105422. https://doi.org/10.1016/j.orgel.2019.105422
Cheung HC (1991) Resonance energy transfer, topics in fluorescence spectroscopy: principles. Springer US, Boston, MA
Watrob HM, Pan CP, Barkley MD (2003) Two-step FRET as a structural tool. J Am Chem Soc 125(24):7336–7343. https://doi.org/10.1021/ja034564p
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4(6):435–446. https://doi.org/10.1038/nmat1390
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5(9):763–775. https://doi.org/10.1038/nmeth.1248
Klostranec JM, Chan WC (2006) Quantum dots in biological and biomedical research: recent progress and present challenges. Adv Mater 18(15):1953–1964. https://doi.org/10.1002/adma.200500786
Bhattacharyya S, Jana B, Patra A (2015) Multichromophoric organic molecules encapsulated in polymer nanoparticles for artificial light harvesting. ChemPhysChem 16(4):796–804. https://doi.org/10.1002/cphc.201402723
Zhao Q, Chen Y, Li SH, Liu Y (2018) Tunable white-light emission by supramolecular self-sorting in highly swollen hydrogels. Chem Commun 54(2):200–203. https://doi.org/10.1039/C7CC08822A
Pallavi P, Sk B, Ahir P, Patra A (2018) Tuning the Förster Resonance Energy Transfer through a Self-Assembly Approach for efficient White‐Light Emission in an aqueous medium. Chemistry–A Eur J 24(5):1151–1158. https://doi.org/10.1002/chem.201704437
Clark RJH, Hester RE (eds) (1996) Advances in Spectroscopy. Wiley, New York. https://doi.org/10.1016/S1631-0748(02)01341-3
Csele MS, Engs P (2004) Fundamentals of Light and Lasers. Wiley, New York
Drake JM, Klafter J, Levitz P (1991) Chemical and biological microstructures as probed by dynamic processes. Science 251(5001):1574–1579. https://doi.org/10.1126/science.2011737
Yılmaz Y, Erzan A, Pekcan Ö (1998) Critical exponents and fractal dimension at the sol-gel phase transition via in situ fluorescence experiments. Phys Rev E 58(6):7487. https://doi.org/10.1103/PhysRevE.58.7487
Huamán AA, Celestino MR, Quintana ME (2021) Theoretical and experimental study of solar cells based on nanostructured films of TiO2 sensitized with natural dyes extracted from Zea mays and Bixa orellana. RSC Adv 11(16):9086–9097. https://doi.org/10.1039/D1RA01043C
Diallo A, Zongo S, Mthunzi P, Rehman S, Alqaradawi SY, Soboyejo W, Maaza M (2014) Z-scan and optical limiting properties of Hibiscus Sabdariffa dye. Appl Phys B 117:861–867. https://doi.org/10.1007/s00340-014-5900-4
Wang LS, Lee CT, Su WL, Huang SC, Wang SC (2016) Delonix regia leaf extract (DRLE): a potential therapeutic agent for cardioprotection. PLoS ONE 11(12):e0167768. https://doi.org/10.1371/journal.pone.0167768
El-Sayed AM, Ezzat SM, Salama MM, Sleem AA (2011) Hepatoprotective and cytotoxic activities of Delonix regia flower extracts. Pharmacognosy J 3(19):49–56. https://doi.org/10.5530/pj.2011.19.10
Modi A, Mishra V et al (2016) Delonix regia: historic perspectives and modern phytochemical and pharmacological researches. Chin J Nat Med 14(1):31–39. https://doi.org/10.3724/SP.J.1009.2016.00031
Khoo HE, Azlan A, Tang ST, Lim SM (2017) Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. https://doi.org/10.1080/16546628.2017.1361779
Ebada D, Hefnawy HT, Gomaa A, Alghamdi AM, Alharbi AA, Almuhayawi MS, Namir M (2023) Characterization of Delonix regia flowers’ pigment and polysaccharides: evaluating their antibacterial, anticancer, and antioxidant activities and their application as a natural colorant and sweetener in beverages. Molecules 28(7):3243. https://doi.org/10.3390/molecules28073243
Khongkaew P, Wattanaarsakit P, Papadopoulos KI, Chaemsawang W (2021) Antioxidant effects and in vitro cytotoxicity on Human Cancer Cell lines of Flavonoid-Rich Flamboyant (Delonix regia (Bojer) Raf.) Flower Extract. Curr Pharm Biotechnol 22(13):1821–1831. https://doi.org/10.2174/1389201021666201029154746
Nayak R, Houshyar S, Khandual A, Padhye R, Fergusson S (2020) Identification of natural textile fibres. In handbook of natural fibres. Woodhead Publishing, pp 503–534. https://doi.org/10.1016/B978-0-12-818398-4.00016-5
Kurniawan YS, Fahmi MR, Yuliati L (2020) Isolation and optical properties of natural pigments from purple mangosteen peels. IOP Conf Ser Mater Sci Eng IOP Publishing 833(1):012018. https://doi.org/10.1088/1757-899X/833/1/012018
Drah A, Tomić NZ, Veličić Z et al (2017) Highly ordered macroporous γ-alumina prepared by a modified sol-gel method with a PMMA microsphere template for enhanced Pb2+, Ni2+ and Cd2+ removal. Ceram Int 43(16):13817–13827. https://doi.org/10.1016/j.ceramint.2017.07.102
Merlin JC, Statoua A, Cornard JP, Saidi-Idrissi M, Brouillard R (1993) Resonance Raman spectroscopic studies of anthocyanins and anthocyanidins in aqueous solutions. Phytochemistry 35(1):227–232. https://doi.org/10.1016/S0031-9422(00)90539-9
Nickless EM, Holroyd SE, Stephens JM, Gordon KC, Wargent JJ (2014) Analytical FT-Raman spectroscopy to chemotype Leptospermum scoparium and generate predictive models for screening for dihydroxyacetone levels in floral nectar. J Raman Spectrosc 45(10):890–894. https://doi.org/10.1002/jrs.4576
Harborne JB (1967) Comparative biochemistry on the flavonoids. Academic Press. London and New York.
Zumahi A-A et al (2020) Extraction, optical properties, and aging studies of natural pigments of various flower plants. Heliyon 6(9):e05104. https://doi.org/10.1016/j.heliyon.2020.e05104
Michels L, Richter A, Chellappan RK, Røst HI, Behsen A, Wells KH, Blawid S (2021) Electronic and structural properties of the natural dyes curcumin, bixin and indigo. RSC Adv 11(23):14169–14177. https://doi.org/10.1039/d0ra08474c
Felicissimo MP, Bittencourt C, Houssiau L, Pireaux JJ (2004) Time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy analyses of Bixa orellana seeds. J Agric Food Chem 52(7):1810–1814. https://doi.org/10.1021/jf035027r
Wang P, Liu J, Zhuang Y, Fei P (2022) Acylating blueberry anthocyanins with fatty acids: improvement of their lipid solubility and antioxidant activities. Food Chemistry: X 15:100420. https://doi.org/10.1016/j.fochx.2022.100420
Godibo DJ, Anshebo ST, Anshebo TY (2015) Dye sensitized solar cells using natural pigments from five plants and quasi-solid state electrolyte. J Braz Chem Soc 26:92–101. https://doi.org/10.5935/0103-5053.20140218
Vettumperumal R, Kalyanaraman S, Selvan GT, Selvakumar PM (2018) Fluorescence analysis of natural dyes from Plumeria rubra (red and white) flowers. Optik 159:108–114. https://doi.org/10.1016/j.ijleo.2018.01.070
Picollo M, Aceto M, Vitorino T (2019) UV-Vis spectroscopy. Phys Sci Reviews 4(4):20180008. https://doi.org/10.1515/psr-2018-0008
Renuka CG (2020) Solvatochromism and electronic structure of coumarin derivative. AIP Conf Proc AIP Publishing 2220(1):130022. https://doi.org/10.1063/5.0001777
Belay A (2010) Measurement of integrated absorption cross-section, oscillator strength and number density of caffeine in coffee beans by integrated absorption coefficient technique. Food Chem 121(2):585–590. https://doi.org/10.1016/j.foodchem.2009.12.052
Rybczynski P, Kaczmarek-Kȩdziera A (2021) BODIPY dimers: structure, interaction, and absorption spectrum. Struct Chem 32:953–965. https://doi.org/10.1007/s11224-021-01759-1
Tauc J (ed) (2012) Amorphous and liquid semiconductors. Springer Science & Business Media, Germany
Yu PY, Cardona M (1996) Fundamentals of semiconductors. Springer, 3-540-USA
Fox M (2002) Optical properties of solids. Oxford Univ. Press, England
Sze SM (1981) Physics of semiconductor devices. Wiley, New York
Amri A, Hasan K et al (2019) Surface structural features and optical analysis of nanostructured Cu-oxide thin film coatings coated via the sol-gel dip coating method. Ceram Int 45(10):12888–12894. https://doi.org/10.1016/j.ceramint.2019.03.213
Kinoshita S, Nishi N, Saitoh A, Kushida T (1987) Urbach tail of organic dyes in solution. J Phys Soc Jpn 56(11):4162–4175. https://doi.org/10.1143/JPSJ.56.4162
Ebrahimi S, Yarmand B (2020) Solvothermal growth of aligned SnxZn1-xS thin films for tunable and highly response self-powered UV detectors. J Alloys Compd 827:154246. https://doi.org/10.1016/j.jallcom.2020.154246
Duan L, Yi H, Zhang Y et al (2019) Comparative study of light-and thermal-induced degradation for both fullerene and non-fullerene-based organic solar cells. Sustain Energy Fuels 3(3):723–735. https://doi.org/10.1039/C8SE00567B
Amin PO, Muhammadsharif FF, Saeed SR, Sulaiman K (2021) A study on optoelectronic parameters of natural dyes extracted from beetroot, cabbage, walnut, and henna for potential applications in organic electronics. J Fluoresc 32:203–213. https://doi.org/10.1007/s10895-021-02837-7
Shaaban ER, Kansal I, Mohamed SH, Ferreira JM (2009) Microstructural parameters and optical constants of ZnTe thin films with various thicknesses. Physica B 404(20):3571–3576. https://doi.org/10.1016/j.physb.2009.06.002
Pawlicki M, Collins HA, Denning RG, Anderson HL (2009) Two-photon absorption and the design of two‐photon dyes. Angew Chem Int Ed 48(18):3244–3266. https://doi.org/10.1002/anie.200805257
Duarte DA, Massi M, da Silva Sobrinho AS (2014) Development of dye-sensitized solar cells with sputtered N-doped thin films: from modeling the growth mechanism of the films to fabrication of the solar cells. Int J Photoenergy. https://doi.org/10.1155/2014/839757
Amin PO, Kadhim AJ, Ameen MA, Abdulwahid RT (2018) Structural and optical properties of thermally annealed Ti2 – SiO2 binary thin films synthesized by sol–gel method. J Mater Sci: Mater Electron 29:16010–16020. https://doi.org/10.1007/s10854-018-9688-6
Sharma A, Aggarwal S (2018) Optical investigation of soda lime glass with buried silver nanoparticles synthesised by ion implantation. J Non-cryst Solids 485:57–65. https://doi.org/10.1016/j.jnoncrysol.2018.01.038
Sakr GB, Yahia IS, Fadel M, Fouad SS, Romčević N (2010) Optical spectroscopy, optical conductivity, dielectric properties and new methods for determining the gap states of CuSe thin films. J Alloys Compd 507(2):557–562. https://doi.org/10.1016/j.jallcom.2010.08.022
Yahia IS, Zahran HY, Alamri FH (2016) Pyronin Y as new organic semiconductors: structure, optical spectroscopy and electrical/dielectric properties. Synth Met 218:19–26. https://doi.org/10.1016/j.synthmet.2016.04.024
Pereira TM, Vitório F, Amaral RC, Zanoni KPS, Iha NYM, Kümmerle AE (2016) Microwave-assisted synthesis and photophysical studies of novel fluorescent N-acyl hydrazone and semicarbazone-7-OH-coumarin dyes. New J Chem 40(10):8846–8854. https://doi.org/10.1039/C6NJ01532H
Bindhu CV, Harilal SS, Nampoori VPN, Vallabhan CPG (1999) Solvent effect on absolute fluorescence quantum yield of rhodamine 6G determined using transient thermal lens technique. Mod Phys Lett B 13(16):563–576. https://doi.org/10.1142/S0217984999000725
Choi J, Kim SH, Lee W, Yoon C, Kim JP (2012) Synthesis and characterization of thermally stable dyes with improved optical properties for dye-based LCD colour filters. New J Chem 36(3):812–818. https://doi.org/10.1039/C2NJ20938A
De Lima SR, Felisbino DG, Lima MR, Chang R, Martins MM, Goulart LR, Pilla V (2019) Fluorescence quantum yield of natural dye extracted from Tradescantia pallida purpurea as a function of the seasons: preliminary bioapplication as a fungicide probe for necrotrophic fungi. J Photochem Photobiol B 200:111631. https://doi.org/10.1016/j.jphotobiol.2019.111631
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, USA
Birks JB (1970) Photophysics of aromatic molecules. Wiley-Interscience, New York
Deng Y, Li S et al (2020) Synthesis and a photo-stability study of organic dyes for electro-fluidic display. Micromachines 11(1):81. https://doi.org/10.3390/mi11010081
Luo Q, Zhang H, Zhao Y, Wang J, Yu T (2018) Synthesis and characterization of 9, 10-[di-p-(7-diethylamino-coumarin-3-yl) thiopheneyl] anthracene as fluorescent material. J Sulfur Chem 39(1):89–98. https://doi.org/10.1080/17415993.2017.1391813
Knox RS (1996) Electronic excitation transfer in the photosynthetic unit: reflections on work of William Arnold. Photosynth Res 48:35–39. https://doi.org/10.1007/BF00040993
Clegg RM (1995) Fluorescence resonance energy transfer. Curr Opin Biotechnol 6(1):103–110. https://doi.org/10.1016/0958-1669(95)80016-6
Renuka CG (2020) Fluorescence lifetime decay study of 2, 3, 6, 7-tetrahydro-1H, 5H, 11H- [1] benzopyrano [6, 7, 8-ij]-quinoliz-11-one molecule: As a function of solvent viscosity and temperature. AIP Conf Proc AIP Publishing 2220(1):140017. https://doi.org/10.1063/5.0001787
Raikar US, Renuka CG, Nadaf YF, Mulimani BG, Karguppikar AM, Soudagar MK (2006) Solvent effects on the absorption and fluorescence spectra of coumarins 6 and 7 molecules: determination of ground and excited state dipole moment. Spectrochim Acta Part A Mol Biomol Spectrosc 65(3–4):673–677. https://doi.org/10.1016/j.saa.2005.12.028
Satpati AK, Kumbhakar M, Nath S, Pal H (2009) Photophysical properties of Coumarin-7 dye: role of twisted intramolecular charge transfer state in high polarity Protic solvents. Photochem Photobiol 85(1):119–129. https://doi.org/10.1111/j.1751-1097.2008.00405.x
Haugland RP, Yguerabide J, Stryer L (1969) Dependence of the kinetics of singlet-singlet energy transfer on spectral overlap. Proc Natl Acad Sci 63(1):23–30. https://doi.org/10.1073/pnas.63.1.23
Rolinski OJ, Birch DJ (2000) Determination of acceptor distribution from fluorescence resonance energy transfer: theory and simulation. J Chem Phys 112(20):8923–8933. https://doi.org/10.1063/1.481506
Stryer L (1978) Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem 47(1):819–846. https://doi.org/10.1146/annurev.bi.47.070178.004131
Hegde PK, Adhikari AV, Manjunatha MG, Sandeep CS, Philip R (2009) Synthesis and nonlinear optical characterization of new poly {2, 2′-(3, 4-didodecyloxythiophene-2, 5-diyl) bis [5-(2-thienyl)-1, 3, 4-oxadiazole]}. Synth Met 159(11):1099–1105. https://doi.org/10.1016/j.synthmet.2009.01.034
Yekta A, Duhamel J, Winnik MA (1995) Dipole-dipole electronic energy transfer. Fluorescence decay functions for arbitrary distributions of donors and acceptors: systems with planar geometry. Chem Phys Lett 235(1–2):119–125. https://doi.org/10.1016/0009-2614(95)00045-6
Babu KV, Renuka CG, Nagabhushana H (2018) Mixed fuel approach for the fabrication of TiO2: Ce3+ (1–9 mol%) nanophosphors: applications towards wLED and latent finger print detection. Ceram Int 44(7):7618–7628. https://doi.org/10.1016/j.ceramint.2018.01.184
Kumar S, Puttaraju B, Patil S (2016) A deep-blue Electroluminescent device based on a Coumarin Derivative. ChemPlusChem 81(4):384–390. https://doi.org/10.1002/cplu.201500572
McCamy CS (1992) Correlated colour temperature as an explicit function of chromaticity coordinates. Colour Res Application 17(2):142–144. https://doi.org/10.1002/col.5080170211
Ghosh A, Selvaraj P, Sundaram S, Mallick TK (2018) The colour rendering index and correlated colour temperature of dye-sensitized solar cell for adaptive glazing application. Sol Energy 163:537–544. https://doi.org/10.1016/j.solener.2018.02.021
Wang Z, Yang Z et al (2018) Red phosphor Rb2NbOF5: Mn4+ for warm white light-emitting diodes with a high colour-rendering index. Inorg Chem 58(1):456–461. https://doi.org/10.1021/acs.inorgchem.8b02676
Huang Y, Cohen TA, Luscombe CK (2022) Naturally derived Organic dyes for LED lightings of high Color Rendering and Fidelity Index. Adv Sustainable Syst 6(2):2000300. https://doi.org/10.1002/adsu.202000300
Li C, Cui G et al (2016) Accurate method for computing correlated colour temperature. Opt Express 24(13):14066–14078. https://doi.org/10.1364/OE.24.014066
Pramod AG, Nadaf YF, Renuka CG (2019) A combined experimental theoretical approach for energy gap determination, photophysical, photostable, optoelectronic, NLO, and organic light emitting diode (OLED) application: synthesized coumarin derivative. J Mol Struct 1194:271–283. https://doi.org/10.1016/j.molstruc.2019.05.099
Peng S, Zhao Y et al (2018) Acquiring high-performance deep‐blue OLED emitters through an unexpected blueshift colour‐tuning effect induced by electron‐donating‐OMe substituents. Chemistry–A Eur J 24(32):8056–8060. https://doi.org/10.1002/chem.201800974
Shi J, Xu L et al (2019) Efficient and colour-purity blue electroluminescence by manipulating the coupling forms of D–A hybrids with phenothiazine as the strong donor. Dyes Pigm 160:962–970. https://doi.org/10.1016/j.dyepig.2018.08.055
Sanju KS, Neelakandan PP, Ramaiah D (2011) DNA-assisted white light emission through FRET. Chem Commun 47(4):1288–1290. https://doi.org/10.1039/C0CC04173D
Ledemi Y, Trudel A et al (2014) White light and multicolour emission tuning in triply doped Yb 3+/Tm 3+/Er 3+ novel fluoro-phosphate transparent glass-ceramics. J Mater Chem C 2(25):5046–5056. https://doi.org/10.1039/C4TC00455H
Xia Z, Liu Q (2016) Progress in discovery and structural design of colour conversion phosphors for LEDs. Prog Mater Sci 84:59–117. https://doi.org/10.1016/j.pmatsci.2016.09.007
McCluney WR (2014) Introduction to radiometry and photometry. Artech House
Franc C, Grum (1979) Optical Radiation measurements (v. 1). Academic, New York, New York
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Harshitha. D: Writing – review & editing, Writing Original draft, visualization, validation. Anil Kumar: Calculations - Förster Resonance Energy transfer parameters. Mahesh H. M: Analysis- UV-Visible spectroscopy.C.G. Renuka: Writing – review & editing, Supervision, Data curation, visualization, validation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harshitha, D., Kumar, A., Mahesh, H.M. et al. Anthocyanins of Delonix Regia Floral Petals: A Novel Approach on Fluorescence Enhancement, Forster Resonance Energy Transfer Mechanism and Photostability Studies for Optoelectronic Applications. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03730-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03730-9