Abstract
Lithium-aluminum-borate glasses doped with single, double, or triple Tm3+, Tb3+, or Eu3+ ions, respectively, at several concentrations were prepared. Structural characterization was performed using optical absorption and luminescence spectroscopy. The transition for the rare earth ions under study was observed in optical absorption and some for-host glass at wavelengths less than 300 nm. The luminescence of Eu3+, Tb3+, and Tm3+ is presented as bright red, green, and blue emissions, respectively. White light was produced by adjusting the excitation wavelength and doping concentration of Tm2O3, Eu2O3, and Tb4O7. The sample doped with triple rare earth ions produced color emissions from Tb and Tm to Eu, which varied in hue based on the excitation wavelengths of 350, 360, and 370 nm. The glass samples under investigation may be promising for optoelectronic devices and security applications such as data encryption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Researchers have shown significant interest in glass samples doped with rare-earth ions for various applications. Such as color displays, a developing field in medical devices, high-density optical data storage and reading, laser optical fibers, optical amplifiers, and visible display devices [1,2,3,4,5,6,7]. Owing to the internal shielding of electrons by the 5 s and 5p shells, the strong transitions within the 4fN of the rare-earth ions that cause it to occur are not influenced by any external fields [8, 9]. According to research on various glass systems that serve as hosts for rare earth ions [10,11,12,13,14,15,16], borate glass is a promising material owing to its high transparency, broad range of formation, thermal and mechanical stability, and high solubility of lanthanum ions. Using the melt-quenching process, single- and double-doped (Tb3+ and Eu3+) lithium borate glass was created, and the photoluminescence properties of the glass samples were investigated. The CIE values change from warm red to cool white [17]. For the first time, borate glass doped with Ce3+, Tb3+, and Mn2+ produced white light when excited by UV light [18]. It took time and effort to develop a single-host glass system that emitted white light. Lin et al. [19] found that SiO2-Al2O3-CaO-CaF2 glass was excited by ultraviolet light and doped with Tb/Sm. Furthermore, Zhu et al. [20] produced Tm/Tb/Sm silicate glass at wavelengths below those of the near-ultraviolet excitation. However, it failed to provide the expected outcomes.
The melt quenching technique was used to produce the glass samples CaO-Al2O3-B2O3-RE2O3 (RE = Eu, Tb, and Tm). The photoluminescence of these glass samples was investigated, and mixed bands of blue, green, and reddish-orange light were observed in their emission spectra [21]. Samples under 360 nm excitation emit white light. In addition, the energy transfer (ET) between Tb3+ and Eu3+ ions in triply doped glasses were validated. Hence, glass samples are potential candidates for white LEDs.
The characteristics of the glass samples were susceptible to variations in the Tm2O3 concentration. According to the findings of this study, glass samples may be beneficial for laser and communication applications [22].
The goal was to prepare samples for a glass system that produces white light by mixing an appropriate combination of blue, green, and red emissions. Tm2O3, Tb4O7, and Eu2O3 were anesthetized in one glass group. By adding, first, each element separately, then, second, two elements together, and finally, all three elements are combined. A series of glass samples containing one rare earth ion (Tm or Tb or Eu), two rare earth ions (Tm + Tb or Tm + Eu or Tb + Eu), or three rare earth ions (Tm + Tb + Eu) were prepared. The photoluminescence behavior of the single-, double-, and triple-doped glass samples was examined. We analyzed and investigated various lanthanide energy transfer processes that occur in various doped glass samples. The effect of adding an equal amount of double and triple rare earth ions on photoluminescence spectra (PL) was also studied. It also shows how the intensity of the excitation energy can control the color change of radiation emitted by the sample.
Experimental Work
Glass samples were prepared according to Table 1 composition. To remove the hydrogen and carbon contained in the raw materials, the samples were held for 30 min at 400 °C and then melted at 1000 °C for an hour. A high-temperature liquid (1000 °C) was poured between the two copper plates at room temperature (quenching melt technique).
A Philips Analytical X-ray diffraction system, type PW3710, based on a Cu tube anode with wavelengths of Kα1 = 1.54060 Å and Kα2 = 1.54439°A, was used to confirm the glassy samples. The generator current was 30 mA, the generator tension was 40 kV. 10o was the start angle (2θ) and 70o was the end angle. To measure the optical transmission spectra, a computerized recording spectrophotometer was used to measure the wavelengths between 190 and 2500 nm (JASCO, V-570). Room-temperature emission spectra were obtained using a 150 W Xenon arc lamp and a JASCO FP-8300 spectrofluorometer.
Results and Discussion
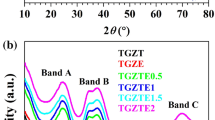
Figure 1 shows the X-ray diffraction patterns of the studied samples. The two broad peaks at 2θ equal 23°and 43°are the diffracted X-ray peaks from the amorphous network of the rare-earth-doped glass. The broad diffraction peaks indicate disordered long-range order arrangements in the amorphous state of the studied samples. An X-ray diffraction pattern with a broader peak may signify a smaller crystal, a fault in the crystalline structure, or even an amorphous sample that lacks perfect crystalline. The Scherrer equation indicates that increased broadening will be caused by lower crystal sizes. Peak width and crystal size are inversely correlated. A thinner peak is related to a larger crystal. A broader peak indicates the presence of a smaller crystal or an amorphous material.
Figure 2 shows the absorption spectra of the glass samples doped with various RE ions. Figure 2 shows the transition in the RE brought about by 4f-4f as well as the lower absorption and high transmission anticipated at a certain wavelength. The transition that evolved in the host glass may be the cause of the absorption peaks below 350 nm [23]. The transition from the ground state 3H6 to several excited states is visible in the glass sample doped with Tm2O3 [24, 25]. At 350, 456, 686, 790, 1202, and 1648 nm, for 1D2, 1G4, 3F3 + 3F2, 3H4, 3H5, and 3F4 are the corresponding wavelengths. Broad peaks in the near-IR region, which correspond to the 7F6 → 7F0, 7F1, and 7F2 transitions at 1906, 1870, and 1756 nm and 7F6 → 7F3 at 2174 nm, are visible in the sample doped with Tb4O7 [26,27,28]. However, the sample doped with Eu2O3 exhibits a transition between the two ground states, 7F0 and 7F1 (the lower energy difference between them is approximately 200 cm−1). The anticipated transitions at 376, 392, 464, 528, 2108, and 2206 nm correspond to 7F0 → 5G2, 7F0 → 5L6, 7F0 → 5D2, 7F0 → 15D1, 7F0 → 7F6, and 7F1 → 7F6 transition [29, 30].
The absorption spectra of the double- and triple-rare-earth ion-doped glass samples are shown in Figs. 3 and 4, respectively. The doped double or triple RE in the glass sample exhibits the appearance of mixing peaks for each RE, as can be seen from these figures.
The absorption spectra of the double rare-earth ion-doped glass samples are shown in Fig. 3. This allowed us to observe the spectra of a material containing Tb3+ and Eu3+ ions, which showed five peaks: two in the visible spectrum at 394 and 372 nm and three in the NIR region at 2200, 2100, and 1940 nm. The middle spectrum of the sample containing Tb3+ and Tm3+ ions exhibited five peaks: three in the visible region at 790, 685, and 465 nm, two in the near-infrared region at 2200 nm, and a broad peak from 1600 to 2050 nm.
Seven peaks can be seen in the lower spectrum of the sample containing Tm3+ and Eu3+: four in the visible spectrum at 790, 685, 460, and 395 nm, three in the NIR region at 2200, 2100, and 1650 nm. The absorption spectra of the triple-rare-earth ion-doped glass samples are shown in Fig. 4. Four peaks in the visible region at 790, 685, 468, and 394 nm, six peaks in the NIR range at 2200, 2100, 1950, 1880, 1700, and 1650 nm are observed in this figure.
The optical bandgap Eg is a crucial metric for describing the structural and optical characteristics of a material [31] and identifying its type [32]. Semiconductor materials have Eg values between 0 and 4 eV. A substance is an insulator if its value exceeds four. For direct and indirect allowed transitions, Eg is calculated by extrapolating the relationship between the (αhv) power 1/2 or 2 on the y-axis and \(hv\) = 0 on the x-axis, as shown by Mott and Davies:
where α is the absorption coefficient, n is the type of transition [33], \(hv\) is the photon energy, and A is a constant. The Eg values determination are shown in Fig. 5a and b.
Table 2 lists the calculated optical band gap values. Compared with these results, the resulting values largely match the semiconductor property [33,34,35,36,37,38,39].
For many different types of photonic devices, the band gap energy is crucial.
The band gap energy has a significant role in determining the emission wavelengths of light emitting diodes and laser diodes. The wavelengths become shorter as the energy level rises. Notably, in conditions with large carrier densities, the highest emission occurs at photon energies that are only a little bit above the band gap energy. This is due to the fact that the density of states drastically increases with frequency.
Only photon energies over the band gap energy can produce a significant response from photodiodes and other semiconductor photodetectors since that is the prerequisite for efficient absorption, which generates the necessary photocurrent. The lower density of states causes the responsivity to typically decline significantly close to the band gap energy.
The host, excitation wavelength, and RE concentration are the three variables that affect the emission of the glass sample.
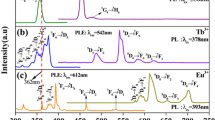
Figure 6A shows the excitation spectrum of the Tm-doped glass sample measured at 452 nm (1D2 → 3F4). The excitation curve displays a high intensity measured at 358 nm, ascribed to 3H6 → 1D2, along with low-intensity peaks in the region of 250–300 nm corresponding to 3H6 → 3P2, 3P1, and 3P0 + 1I6 [23, 40].
The emission spectrum of the sample containing Tm2O3 excited at 358 nm is shown in Fig. 6B. According to the emission spectra, the emission peaks at 454 and 476 nm are attributed to transitions between the two ground states at 3F4 and 3H6 [22] and are designated as 1D2 → 3F4 and 1G4 → 3H6 [23, 40]. Blue emission was linked to a prominent peak at 454 nm [22].
The glass sample containing 1 mol% Tm2O3 had chromatic coordinates of x = 0.155 and y = 0.039, which are in the blue region of Fig. 6C and are in accordance with the literature [41].
The monitoring of the emission sample containing Tb3+ ions at 545 nm is shown in Fig. 6A. Figure 6A depicts the transitions from the ground state of 7F6 to 5H6, 5H7, 5L7, 5L9, 5L10, 5G5 + 5D3, and 5D4 at 300, 317, 340, 352, 369, 378, and 484 nm, respectively [42, 43]. These bands originate from the Tb3+ ion 4f-4f transitions, and the band at 285 nm causes a 4F7–5D1 transition. The bands at 369, 378, and 352 nm were sufficiently intense to attain the required wavelength to excite the glass sample (Fig. 6B). As shown in Fig. 6B, it was possible to investigate emission at 543 nm (5D4–7F5). This emission has a high intensity and is connected to green emissions 5D4 → 7F6, 5D4 → 7F4, and 5D4 → 7F3 respectively [43, 44]. The chromatic coordinates were x = 0.273 and y = 0.574, which are indicated in the green region in Fig. 6C.
The excitation spectra presented in Fig. 6A are the result of the 613 nm emission observed in the glass sample containing Eu2O3. In the excitation spectra, seven peaks were excited from ground states 7F0 and 7F1 to several excited states [7, 18, 19] with wavelengths of 362, 377, 395, 403, 465, 527, and 534 nm, for transition 7F0 → 5D4, 7F0 → 5L7, 7F0 → 5L6, 7F0 → 5D3, 7F0 → 5D2, and 7F0 → 5D1. In the UV range, high intensity was observed at 395 nm.
Emission spectra of glass sample after excitation at 394 nm are shown in Fig. 6B. Figure 6B shows the creation of an emission peak attributed to Eu3+ (4f7–4f7) transitions [45,46,47] at 579, 592, 613, 657, and 702 nm, corresponding to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions [48]. As a result of how the permitted electric dipole [49, 50] acts when it is influenced by a hypersensitive environment, the peak at 613 nm (5D0 → 7F2) recorded a high intensity. The peak at 592 nm (5D0 → 7F1) [50] denotes a magnetic dipole that is permitted, is independent of the local symmetry, and oversees orange emission [42, 51]. As a result, the local environment [52, 53] around Eu equals 1.9, which is higher than unity, describing the Eu3+ ions in the centric sites [54]. The occurrence of a peak at 657 nm (5D0 → 7F3) indicates the blending of magnetic and electric characteristics, highlighting the asymmetric location of Eu3+ ions in the matrix [42], while also utilizing the emission bands to modulate the total emission color of the glass using the 1931 CIE chromatically [54] theory, as shown in Fig. 6C. From Fig. 6C, it can be observed that the sample's X and Y chromatic coordinates are (0.61, 0.35), which depicts the red–orange light emitted by the glass sample [46, 53, 54].
We can analyze the effect of combining rare earth ions on emission and color owing to differences in color emission with different RE ions. Individual RE ions were studied as described in the previous section.
The two main methods of transferring energy are the multipolar interaction mechanism and the exchange mechanism. The distance between rare earth ions typically serves as the barrier between them. It is the exchange contact mechanism when the critical distance Rc is smaller than 4. When it exceeds 4, the multipole interaction mechanism. There are three different forms of multipole interactions among them: electric dipole–dipole, dipole-quadrupole, and quadrupole–quadrupole [55].
Tm and Eu Doped Glass Sample
Figure 7A shows the excitation spectra of glass samples containing equal amounts of Tm2O3 and Eu2O3 for measuring the emission at 452 nm and 613 nm.
The distance between Tm3+ and Eu3+ (Rc) must first be determined to comprehend the energy transfer between them. This distance was determined using the following formula [56]:
where M is the molecular weight of sample, ρ is density, Na is the Avogadro’s number, x is the concentration of rare earth ions (as shown in Table 3).
The values of Rc lead us to believe that the distance between Tm3+ and Eu3+ is higher than 4, and that the multipole interaction mechanism is responsible for their transition.
Upon sample excitation at 394 nm, the typical emission peaks of Eu3+ were observed at 591 nm and 613 nm. However, when the sample was excited at 358 nm, emission peaks for both Tm3+ and Eu3+ with a weak peak at 613 nm were observed (Fig. 7B). The high emission band at approximately 450 nm, which corresponds to the Tm emission, and the weak band at 613 nm, which corresponds to the Eu3+ emission, were visible upon excitation at a Tm wavelength of 358 nm. And energy transfer from Tm3+ to Eu3+ ions. The examination of the ratio of the varied intensities between 5D0 → 7F1,2 could determine the symmetry around Eu3+, even though the Eu excitation wavelength at 394 nm only has the emission peak of Eu3+. High intensity of 5D0 → 7F1 over 5D0 → 7F2. This demonstrates a stronger covalency between Eu3+ and some oxygen [50] and the fact that the bulk of Eu3+ ions occupy inversion-symmetry locations [57]. Figure 8 illustrates how the CIE algorithm used the obtained emission data to shift the obtained color toward the center and determined the color chromatically as (0.17, 0.059) for Tm excitation and (0.52, 0.325) for Eu.
The schematic energy level diagram of Tm3+ and Eu3+ is plotted in Fig. 8. Obviously, the energy gap of Tm3+1D2 → 3F4 transitions (approximately 2.18 × 104 cm−1) is similar to the excitation gaps of Eu3+ (2.15 × 104 cm−1) Eu3+7F0 → 5D2, which could generate a cross-relaxation process between Tm3+ and Eu3+ to effectively improve the population of the 5D2 energy level for Eu3+. The energy transfer process involved is as follows:
From the above analysis, we can infer the possibility of energy transfer between Tm3+ and Eu3+. To verify the energy transfer, the fluorescence spectra of the Tm3+-Eu3+ co-doped host are studied. The emission spectra of the Tm3+-Eu3+ co-doped host are excited at 358 nm and 394 nm, as displayed in Fig. 7. Under the excitation of 394 nm, the emission spectrum can only observe the characteristic emission peaks of Eu3+ at 591 nm and 614 nm. However, the emission peaks of not only Tm3+ but also Eu3+ can be observed at 358 nm excitation.
Tm and Tb Doped Host Glass Sample
The emission spectra of the glass samples had equal amounts of Tm2O3 and Tb4O7 for measuring the emission at 452 nm and 545 nm, as shown in Fig. 9.
The excitation spectra of the glass sample had an equal amount of Tm2O3 and Tb4O7 excited at 369 nm and 358 nm, as shown in Fig. 9.
The values of Rc lead us to believe that the distance between Tm3+ and Tb3+ is higher than 4, and that the multipole interaction mechanism is responsible for their transmission.
As shown in Fig. 9, when Tb3+ ions (0.5 mol%) are co-doped with Tm3+ ions (0.5 mol%) in the host glass, the intensity of the Tm3+ ion (1D2 → 3F4) 459 nm emission bands decrease with the addition of Tb3+ ions. This was attributed to the non-radiative and cross-relaxation mechanisms involved in the energy transfer between the Tm3+ and Tb3+ ions. The coincidence of the excitation peaks, as observed in the blending of the two colors (green and blue) from the prior excitation curves for Tm and Tb. In other words, one RE can emit the other RE at the same excitation wavelength. The emission curve was used to observe the emission of the double RE at the same wavelength. In Fig. 9, the excitation at 358 nm is observed, and the ratio of 453 to 543 (the high-intensity peaks of the two RE) is shown to be 1.1, whereas the excitation at 369 nm is shown to be 0.013, showing an energy transfer between the two RE. It is described as having the ability to transfer energy from Tm to Tb [25]. A high-intensity suitable color corresponding to the excitation wavelength was observed. The data analysis from the CIE program (Fig. 10) shows the shifting of the color towards the center.
This could be attributed to the energy transfer among the Tm3+ and Tb3+ ions with the following possible non-radiative cross-relaxation channels as shown in Fig. 10: 5D4 (Tb3+) + 3H6 (Tm3+) → 7F0 (Tb3+) + 3F3 (Tm3+) quenches Tb3+ ions emission bands,
1D2 (Tm3+) + 7F6 (Tb3+) → 1G4 (Tm3+) + 7F0 (Tb3+) quenches Tm3+ ions emission bands.
Tb and Eu Doped Host Glass
The values of Rc lead us to believe that the distance between Tb3+ and Eu3+ is higher than 4, and that the multipole interaction mechanism is responsible for their transition.
Figure 11 shows a comparison of the emission from a glass sample containing equal amounts of Tb4O7 and Eu2O3 (0.5 mol%) and the emission from the same glass sample containing each element separately (1 mol%) to examine the impact of adding Tb4O7 and Eu2O3 together on the emission produced from a sample containing one element. The graph shows that Tb3+-induced luminescence was less intense when Eu3+ ions were present. The Tb3+ ions were excited from the ground state to higher excited states, relaxed to the 5D state by multi-phonon relaxation, and finally returned to the ground state (5D4 → 7F6, 5, 4) of Tb3+. The energy is either absorbed by Eu3+ during these transitions and excited to a higher energy level (7F0 → 5D1,0) or absorbed by Eu3+ during cross-relaxation, which relaxes non-radiatively to the 5D0 level from 5D1; finally, a radiative transition occurs from 5D0 → 7FJ(J=0–6) where the red–orange emission (5D0 → 7F2) is observed [28]. Figure 11 compares the excitation spectra of the host glass co-doped with Tb3+ and Eu3+ to 615 nm of the host glass with only Eu3+. The co-doped Tb3+/Eu3+ excitation spectra revealed a new optical band at 454 nm (7F6 → 5D4), which was attributed to the absorption of Tb3+ ions by Eu ions.
Figure 12 shows the CIE diagram of the glass sample containing 0.5 mol% Tb4O7 and 0.5 mol% Eu2O3.
Emission bands of Eu3+ were measured under excitation of Tb3+ ions by 543 nm line. The spectrum detected for Tb3+ singly doped glass consists of four characteristic emission bands, which correspond to 5D4 → 7FJ (J = 3–6) transitions of trivalent terbium. When a glass sample is co-doped with Tb3+ and Eu3+ ions, the additional emission bands are observed. The detailed spectroscopic analysis indicates that these emission bands correspond to 5D0 → 7FJ (J = 1–4) transitions of Eu3+. All transitions and energy transfer processes were schematized on the energy level diagrams as shown in Fig. 12.
Tb, Tm, and Eu Ions Doped Host Sample
For the samples doped with three lanthanide ions, Tm2O3, Eu2O3, and Tb4O7, the excitation spectra monitored at 614 nm, 545 nm, and 452 nm are shown in Fig. 13.
In the preceding figures [58, 59], From previous study for sample doped with one rare earth ion Tb or Tm or Eu, the typical excitation bands of Eu3+, Tb3+, and Tm3+ are shown in Fig. 13. Therefore, three excitation peaks were elected to investigate the luminescence effects of Tm3+, Tb3+, and Eu3+. The excitation wavelengths were chosen to be 350, 360, and 370 nm because the excitation spectra shows that there is an overlap between all three excitation peaks from 340 to 385 nm.
Figure 13 shows the emission spectra of a glass sample doped with triple rare-earth ions (Tm, Tb, and Eu) at excitation wavelengths of 350, 360, and 370 nm. This allows the selection of a wavelength within the range of exposure of the glass and displays the ability to emit color in a chromatic diagram.
The emission spectra of glass samples with equal mixtures of Eu, Tb, and Tm at various excitation wavelengths (350, 360, and 370 nm) are shown in Fig. 13. Figure 14 shows that energy can be transferred from (Tm3+ and Tb3+) to Eu3+ ions.
Any color determined by the values of triple stimulation X, Y and Z. and related with its hue, saturation, and luminance. The tristimulus values for a non-monochromatic light source with spectral relative power P(λ) are given by:
which gives the power for each of the three primary colors to match with the color of P(λ), and from the tristimulus values the color chromaticity coordinates × and y can be determined using the following expression.
where R = X + Y + Z.
The luminescent intensity of the emission spectral measurements can be characterized using the CIE 1931 chromaticity diagram. These chromaticity coordinates are not linearly independent of each other since they follow that × + y + z = 1 for all colors; Third component can always be computed from first two. It is therefore only necessary to quote two of the chromaticity coordinates, and these can of course be plotted on a normal two-dimensional graph [60,61,62].
The color matching function was used to coordinate the emission spectra through CIE 1931 color coordination [63, 64]. Table 4 lists and calculates the coordination of the glass samples with equal concentrations of Tm2O3, Tb4O7, and Eu2O3 excited at 350, 360, and 370 nm. The information acquired explains why certain light emissions appear in different colors. A standpoint (0.345,0.372) in the samples stimulated at 350 nm indicates a white color. At 360 nm, the excited state creates a blue hue, and at 370 nm, a green color, as shown in Fig. 15.
The CCT is an important parameter used in determining the distinctive color produced using McCamy's approximation formula given below [65],
where n = (x-xe)/(y-ye) is the inverse slope line and xe = 0.332; ye = 0.186.
The type of light bulb used in various regions can be determined using the Kelvin ranges given below.
-
1.
less than 2000 K: emits a soft glow of light, such as candlelight, ideal for dimly lit spaces where ambient illumination is desired.
-
2.
2000 K-3000 K emits mellow white illumination that frequently appears yellow. Best for living rooms, dining rooms, bedrooms, and outdoor settings.,
-
3.
3100 K-4500 K: produces strong white light and is ideal for vanities, workstations, kitchens, and other areas where task lighting is required.
-
4.
4600 K–6500 K emits a bright amount of blue–white light that is similar to that of daylight and is excellent for use in show spaces and work locations. When very strong illumination is required.
-
5.
6500 K and up to 6500 K Kelvin give off a bright, bluish hue of light often found in commercial locations; they are best for bright task lighting.
The obtained findings displayed the highest values, indicating high brightness. ranging from the white emission that occurs naturally to a cool green emission that is more advantageous for fabricating green solid-state lasers and the green portion of tricolor w-LEDs [66, 67].
Conclusion
Borate glass was produced with single, double, and triple RE3+ doping using melt cooling technology. The glass samples under study were activated by the rare earth oxide production Li2O-Al2O3-B2O3- RE oxide (RE = Tm2O3, Eu2O3, and Tb4O7), generating orange-red, green, and blue light synchronously when the excited wavelength light affected the samples. Glass samples doped with triple rare earth ions (equal proportion 0.33 mol%) produced white emission when excited by light at a wavelength of 350 nm. verified energy transfer from Tm3+ and Tb3+ to Eu3+. Eu3+/Tb3+/Tm3+ tri-doped glass is a potential candidate for the fabrication of white light-emitting diodes, luminous materials, and fluorescent display devices. There are numerous applications for rare earth luminous materials. They can also be utilized to make medical imaging, radar, computer displays, everyday lighting fixtures, and more in the industrial sector in addition to metallurgy and glass production. The use of rare earth-ion luminous materials in healthcare and the energy sector application. Based on its reversible transmittance and photoluminescence manipulation, rare earth ion-doped transparent glass can be employed as a 3D optical information storage and data encryption medium.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kumi-Barimah E, Chen Y, Sharma G, Jha A (2022) Judd-Ofelt analysis, visible to NIR photoluminescence emission under 450 nm and 976 nm excitations and energy transfer of barium fluorotellurite glasses doping with Ho3+, Yb3+, Ho3+:Yb3+. Opt Mater X16:100201. https://doi.org/10.1016/j.omx.2022.10020
Do HPV, Quang VX, Tuyen VP, Thanh LD, Khaidukov NM, Makhov VN, Thanh NT (2019) Sensitization of luminescence from Sm3+ ions in fluoride hosts K2YF and K2GdF5 by doping with Tb3+ ions. J Lumin 209:340–345. https://doi.org/10.1016/j.jlumin.2018.12.057
Tuyen VP, Quang VX, Do PV, Thanh LD, Ca NX, Hoa VX, Tuat LV, Thi LA, Nogami M (2019) An in-depth study of the Judd-Ofelt analysis, spectroscopic properties and energy transfer of Dy3+ in alumino-lithium-telluroborate glasses. J Lumin 210:435–443. https://doi.org/10.1016/j.jlumin.2019.03.009
Kumi-Barimah E, Chen Y, Tenwick R, Al-Murish M, Sherma G, Jha A (2022) Effect of Yb3+ on the structural and visible to near-infrared wavelength photoluminescence properties in Sm3+- Yb3+-codoped barium fluorotellurite glasses. Materials 15:3314. https://doi.org/10.3390/ma15093314
Annapoorani K, Basavapoornima C, Suriya Murthy N, Marimuthu K (2016) Investigations on structural and luminescence behavior of Er3+ doped Lithium Zinc borate glasses for lasers and optical amplifier. J Non Cryst Solids 447:273–282. https://doi.org/10.1016/j.jnoncrysol.2016.06.021
Sontakke AD, Biswas K, Tarafder A, Sen R, Annapurna K (2011) Broadband Er3+ emission in highly nonlinear Bismuth modified Zinc-Borate glasses. Opt Mater Express 1:344–356
Swapna K, Mahamuda S, Venkateswarlu M, Rao AS, Jayasimhadri M, Shakya S, Prakash GV (2015) Visible, up-conversion and NIR (∼1.5 μm) luminescence studies of Er3+doped Zinc Alumino Bismuth Borate glasses. J Lumin 163:55–63. https://doi.org/10.1016/j.jlumin.2015.02.036
Van Uffelen M, Girard S, Goutaland F, Gusarov A, Brichard B, Berghmans F (2004) Gamma radiation effects in Er-doped silica fibers. IEEE Trans Nucl Sci 51:2763–2769
Prabhu NS, Hegde V, Sayyed MI, Şakar E, Kamath SD (2019) Investigations on the physical, structural, optical and photoluminescence behavior of Er3+ ions in lithium zinc fluoroborate glass system. Infrared Phys Technol 98:7–15. https://doi.org/10.1016/j.infrared.2019.02.005
George H, Deopa N, Kaur S, Prasad A, Sreenivasulu M, Jayasimhadri M, Rao A (2019) Judd-Ofelt parametrization and radiative analysis of Dy3+ ions doped Sodium Bismuth Strontium Phosphate glasses. J Lumin 215:16693. https://doi.org/10.1016/j.jlumin.2019.116693
Karki S, Kesavulu C, Kim H, Kaewkhao J, Chanthima N, Kothan S, Kaewjaeng S (2019) Physical, optical and luminescence properties of the Dy3+ doped barium borophosphate glasses. J Non-Cryst Solids 521:119483. https://doi.org/10.1016/j.jnoncrysol.2019.119483
Kıbrıslı O, Ersundu A, Ersundu MÇ (2019) Dy3+ doped tellurite glasses for solid-state lighting: an investigation through physical, thermal, structural and optical spectroscopy studies. J Non-Cryst Solids 513:125–136. https://doi.org/10.1016/j.jnoncrysol.2019.03.020
Hegde V, Viswanath CSD, Mahato KK, Kamath SD (2019) Warm white light and colour tunable characteristics of Dy3+ co-doped with Eu3+ and Pr3+ zinc sodium bismuth borate glasses for solid state lighting applications. Mater Chem Phys 234:369–377. https://doi.org/10.1016/j.matchemphys.2019.05.063
Kaur S, Vishwakarma AK, Deopa N, Prasad A, Jayasimhadri M, Rao A (2018) Spectroscopic studies of Dy3+ doped borate glasses for cool white light generation. Mater Res Bull 104:77–82. https://doi.org/10.1016/j.materresbull.2018.04.002
Rimbach AC, Steudel F, Ahrens B, Schweizer S (2018) Tb3+, Eu3+, and Dy3+ doped lithium borate and lithium aluminoborate glass: glass properties and photoluminescence quantum efficiency. J Non-Cryst Solids 499:380–386. https://doi.org/10.1016/j.materresbull.2018.04.002
Grüne M, Schweizer S (2022) Comparison of Dy3+-doped barium borate and lithium aluminoborate glass. Opt Mater 128:112339. https://doi.org/10.1016/j.optmat.2022.112339
Vijayalakshmi L, Kumar KN, Hwang P (2022) Tailoring ultraviolet-green to white light via energy transfer from Tb3+ - Eu3+ codoped glasses for white light-emitting diodes. Scr Mater 187:97–102. https://doi.org/10.1016/j.scriptamat.2020.06.014
Zhang JC, Parent C, Le Flem G, Hagenmuller P (1991) White light emitting glasses. J Solid State Chem 93:17–29. https://doi.org/10.1016/0022-4596(91)90269-N
Lin ZY, Liang XL, Ou YW, Fan CX, Yuan SL, Zeng HD, Chen GR (2010) Full color photoluminescence of Tb3+/Sm3+ codoped oxyfluoride aluminosilicate glasses and glass ceramics for white light emitting diodes. J Alloys Compd 496:L33–L37. https://doi.org/10.1016/j.jallcom.2010.02.155
Zhu CF, Liang XL, Yang YX, Chen GR (2010) Luminescence properties of Tb doped and Tm/Tb/Sm co-doped glasses for LED applications. J Lumin 130:74–77. https://doi.org/10.1016/j.jlumin.2009.07.023
Zhao C, Cai J, Li R, Tie S, Wan X, Shen J (2012) White light emission from Eu3+/Tb3+/Tm3+ triply-doped aluminoborate glass excited by UV light. J Non-Cryst Solids 358:604–608. https://doi.org/10.1016/j.jnoncrysol.2011.11.011
Mhareba MHA, Almessierea MA, Sayyed MI, Alajerami YSM (2019) Physical, structural, optical and photons attenuation attributes of lithium-magnesium-borate glasses: Role of Tm2O3 doping. Optik - Int J Light Electron Opt 182:821–831. https://doi.org/10.1016/j.ijleo.2019.01.111
Kindrat II, Padlyak BV, Lisiecki R, Adamiv VT (2019) Optical spectroscopy and luminescence properties of a Tm3+-doped LiKB4O7 glass. J Non Cryst Solids 521:119477. https://doi.org/10.1016/j.jnoncrysol.2019.119477
Wang T, Wang S, Zhang H, Zou X, Wei Y, Hu W, Su C (2020) Preparation, luminescence properties and energy transfer of Tm3+ and Tm3+-Eu3+ doped glass-ceramics containing NaY(MoO4)2. J Solid State Chem 284:121184. https://doi.org/10.1016/j.jssc.2020.121184
Sasikala T, Moorthy LR (2014) Photoluminescence properties of singly doped Tm3+ and co-doped Tm3+/Tb3+ ions in tellurite glasses. J Mol Struct 1076:529–534. https://doi.org/10.1016/j.molstruc.2014.07.051
Chen Q, Wang H, Wang G, Yin S (2017) Magneto optical properties of rare earth Tb2O3 doped PbO-Bi2O3-B2O3 glass. J Non Cryst Solids 470(5):99–107
Kumar M, Rao AS (2021) Influence of Tb3+ ions concentration and temperature on lithium bismuth alumino borosilicate glasses for green photonic device applications. Opt Mater (Amst) 120(8):111439. https://doi.org/10.1016/j.jnoncrysol.2017.05.008
Quang VX, Do PV, Ca NX, Thanh LD, Tuyen VP, Tan PM, Hoa VX, Hien NT (2020) Role of modifier ion radius in luminescence enhancement from 5D4 level of Tb3+ ion doped alkali-alumino-telluroborate glasses. J Lumin 221(1):117039. https://doi.org/10.1016/j.jlumin.2020.117039
Lakshminarayana G, Qiu J (2009) Photoluminescence of Eu3+, Tb3+ and Tm3+-doped transparent SiO2–Al2O3–LiF–GdF3 glass ceramics. J Alloys Compd 476:720–727. https://doi.org/10.1016/j.jallcom.2008.09.068
Kassab LR, de Almeida R, da Silva DM, de Assumpção TA, de Araújo CB (2009) Enhanced luminescence of Tb3+/Eu3+ doped tellurium oxide glass containing silver nanostructures. J Appl Phys 105:103505. https://doi.org/10.1063/1.3126489
Babu KV, Cole S (2018) Luminescence properties of Dy3+-doped alkali lead alumino borosilicate glasses. Ceram Int 44(8):9080–9090. https://doi.org/10.1016/j.ceramint.2018.02.115
Shaaban KS, El-Maaref AA, Abdelawwad M, Saddeek YB, Wilke H, Hillmer H (2018) Spectroscopic properties and Judd-Ofelt analysis of Dy3+ ions in molybdenum borosilicate glasses. J Lumin 196:477–484. https://doi.org/10.1016/j.jlumin.2017.12.041
Kaur A, Khan S, Kumar D, Bhatia V, Rao SM, Kaur N, Singh K, Kumar A, Singh SP (2020) Effect of MnO on structural, optical and thermoluminescence properties of lithium borosilicate glasses. J Lumin 219:116872. https://doi.org/10.1016/j.jlumin.2019.116872
Kaur R, Bhatia V, Kumar D, Rao SMD, Singh SP, Kumar A (2019) Physical, structural, optical and thermoluminescence behavior of Dy2O3 doped sodium magnesium borosilicate glasses. Results Phys 12:827–839. https://doi.org/10.1016/j.rinp.2018.12.005
Pawar PP, Munishwar SR, Gedam RS (2017) Intense white light luminescent Dy3+ doped lithium borate glasses for W-LED: A correlation between physical, thermal, structural and optical properties. Solid State Sci 64:41–50. https://doi.org/10.1016/j.solidstatesciences.2016.12.009
Deopa N, Rao AS (2017) Photoluminescence and energy transfer studies of Dy3+ ions doped lithium lead alumino borate glasses for w-LED and laser applications. J Lumin 192:832–841. https://doi.org/10.1016/j.jlumin.2017.07.052
Azizan SA, Hashim S, Razak NA, Mhareb MHA, Alajerami YSM, Tamchek N (2014) Physical and optical properties of Dy3+: Li2O-K2O-B2O3glasses. J Mol Struct 1076:20–25. https://doi.org/10.1016/j.molstruc.2014.07.032
Singh S, Kalia G, Singh K (2015) Effect of intermediate oxide (Y2O3) on thermal, structural and optical properties of lithium borosilicate glasses. J Mol Struct 1086:239–245. https://doi.org/10.1016/j.molstruc.2015.01.031
Seshadri M, Bell MJV, Anjos V, Messaddeq Y (2020) Influence of silver ions in Eu3+ doped glass for efficient reddish-orange and white light generation. J Alloys Compd 838:155548. https://doi.org/10.1016/j.jallcom.2020.155548
Kindrat II, Padlyak BV, Lisiecki R, Adamiv VT (2020) Spectroscopic and luminescent properties of the lithium tetraborate glass co-doped with Tm and Ag. J Lumin 225:117357. https://doi.org/10.1016/j.jallcom.2020.157321
El-Maaref AA, Wahab EA, Shaaban KS, Abdelawwad M, Koubisy MSI, Börcsök J, Yousef ES (2020) Visible and mid-infrared spectral emissions and radiative rates calculations of Tm3+ doped BBLC glass. Spectrochim Acta - Part A Mol Biomol Spectrosc 242:118774. https://doi.org/10.1016/j.saa.2020.118774
Zagrai M, Suciu RC, Rada S, Pică ME, Pruneanu S (2021) Structural and optical properties of Eu3+ ions in lead glass for photonic applications. J Non Cryst Solids 569:120988. https://doi.org/10.1016/j.jnoncrysol.2021.120988
Dillip GR, Reddy CM, Rajesh M, Chaurasia S, Raju BDP, Joo SW (2016) Green fluorescence of terbium ions in lithium fluoroborate glasses for fibre lasers and display devices. Bull Mater Sci 39(3):711–717. https://doi.org/10.1007/s12034-016-1192-0
Carreira JFC, Sava BA, Boroica L, Elisa M, Stefan R, Monteiro RCC, Monteiro T, Rino L (2019) Structural and luminescence characterization of a Dy/Tb co-doped borophosphate glass. J Non Cryst Solids 526:119719. https://doi.org/10.1016/j.jnoncrysol.2019.119719
Sales TO, Amjad RJ, Jacinto C, Dousti MR (2019) Concentration dependent luminescence and cross-relaxation energy transfers in Tb3+ doped fluoroborate glasses. J Lumin 205:282–286. https://doi.org/10.1016/j.jlumin.2018.09.031
Jiang F, Sha S, Li S, Xu S, Xu H, Mei X, Zhang Y (2021) Luminescence and structural properties of Eu3+-doped calcium fluoride-bismuth oxide-phosphate glasses. Ceram Int 47(10):13776–13782. https://doi.org/10.1016/j.ceramint.2021.01.241
Zhou D, Wang R, He X, Yi J, Song Zhiguo, Yang Zhengwen, Xuhui X, Xue Y (2015) Color-tunable luminescence of Eu3+ in PbF2 embedded in oxyfluoroborate glass and its nanocrystalline glass. J Alloys Compd 621:62–65. https://doi.org/10.1016/j.jallcom.2014.09.194
Zhao S, Zhu C, Zhou Y, Zhao J (2020) Influence of boron content on the properties of Tm/Eu activated Sr3(PO4), 2–x(BO3)x phosphors. Vacuum 176(2020):109328. https://doi.org/10.1016/j.vacuum.2020.109328
Zhang X, Zhang J, Zhou C, Sun Y, Li P, Qi X (2022) High refractive index of Eu3+ doped La2O3-TiO2-Nb2O5-WO3 oxide glasses with low wavelength dispersion. J Non Cryst Solids 581:121228. https://doi.org/10.1016/j.jnoncrysol.2021.121228
Zhu C, Chaussedent S, Liu S, Zhang Y, Monteil A, Gaumer N, Yue Y (2013) Composition dependence of luminescence of Eu and Eu/Tb doped silicate glasses for LED applications. J Alloys Compd 555:232–236. https://doi.org/10.1016/j.jallcom.2012.12.052
Kaewkhao J, Boonin K, Yasaka P, Kim HJ (2015) Optical and luminescence characteristics of Eu3+ doped zinc bismuth borate (ZBB) glasses for red emitting device. Mater Res Bull 71:37–41. https://doi.org/10.1016/j.materresbull.2015.07.002
Aryal P, Kesavulu CR, Kim HJ, Lee SW, Kang Sang Jun, Kaewkhao J, Chanthima N, Damdee B (2018) Optical and luminescence characteristics of Eu3+-doped B2O3:SiO2:Y2O3:CaO glasses for visible red laser and scintillation material applications. J Rare Earths 36(5):482–491. https://doi.org/10.1016/j.jre.2017.09.017
Bajaj R, Prasad A, Yeswanth AVS, Rohilla P, Kaur S, Rao AS (2022) Down-shifting photoluminescence studies of thermally stable Dy3+ ions doped borosilicate glasses for optoelectronic device applications. J Mater Sci Mater Electron 33(8):4782–4793. https://doi.org/10.1007/s10854-021-07667-8
Ramesh P, Hegde V, Pramod AG, Eraiah B, Agarkov DA, Eliseeva GM, Pandey MK, Annapurna K, Jagannath G, Kokila MK (2020) Compositional dependence of red photoluminescence of Eu3+ ions in lead and bismuth containing borate glasses. Solid State Sci 107:106360. https://doi.org/10.1016/j.solidstatesciences.2020.106360
Kashif I, Ratep A (2022) Judd-Ofelt and luminescence study of Dysprosium-doped lithium borosilicate glasses for lasers and w-LEDs. Boletín de la Sociedad Española de Cerámica y Vidrio 6(1):622–633. https://doi.org/10.1016/j.bsecv.2021.06.001
Kashif I, Ratep A (2023) Influence of dysprosium oxide on physical and optical characteristics of zinc boro-tellurite glasses for optoelectronic device application. Results Opt 11:100401. https://doi.org/10.1016/j.rio.2023.100401
Kłonkowski AM, Wiczk W, Ryl J, Szczodrowski K, Wileńska D (2017) A white phosphor based on oxyfluoride nano-glass-ceramics co-doped with Eu3+ and Tb3+: Energy transfer study. J Alloys Compd 724:649–658. https://doi.org/10.1016/j.jallcom.2017.07.055
Liu Y, Liu GX, Wang JX, Dong XT, Yu WS (2014) Single-component and warm white-emitting phosphor NaGd (WO4)2:Tm3+, Dy3+, Eu3+: synthesis, Luminescence, Energy Transfer, and Tunable Color. Inorg Chem 53(21):11457–11466. https://doi.org/10.1021/ic501284y
Wang D, Chen M, Cheng N, Zhongmin Su, Li C, Zeng F, Zhou Y (2022) Study on luminescence properties of Tm3+/Tb3+/Eu3+ doped germanesilicate glass. J Non-Cryst Solids 584:121504. https://doi.org/10.1016/j.jnoncrysol.2022.121504
Bouman CA (2023) Digital image processing I. School of Electrical and Computer Engineering, Purdue University (Course Information)
Malacara D (2011) Color Vision and Colorimetry: Theory and Applications, 2nd edn. SPIE Press, Bellingham, Washington, USA
Mortimer RJ, Varley TS (2011) Quantifcation of colour stimuli through the calculation of CIE chromaticity coordinates and luminance data for application to in situ colorimetry studies of electrochromic materials. Displays 32:35
Khan I, Rooh G, Rajaramakrishna R, Sirsittipokakun N, Kim SHJ, Kothan S, Kaewkhao J, Kirdsiri K (2020) Comparative study of optical and luminescence properties of Sm3+-ions doped Li2O–Gd2O3–PbO–SiO2 and Li2O-GdF3-PbO–SiO glasses for orange emission solid state device application. J Lumin 222:11713. https://doi.org/10.1016/j.jlumin.2020.117136
Shoaib M, Khan I, Rooh G, Wabaidur SM, Islam MA, Chanthima N, Kothan S, Ullah I, Ahad A, Kaewkhao J (2022) Judd-Ofelt and luminescence properties of Pr3+ doped ZnO-Gd2O3/ GdF3-BaO-P2O3 glasses for visible and NIR applications. J Lumin 247:118884. https://doi.org/10.1016/j.jlumin.2022.118884
Maheshwari K, Rao AS (2022) Photoluminescence downshifting studies of thermally stable Dy3+ ions doped phosphate glasses for photonic device applications. Opt Mater 129:112518. https://doi.org/10.1016/j.optmat.2022.112518
Xu S, Fu J, Liu X, Yuan M, Zhang C, Wang H, Liu G, Cui S (2023) Analysis of thermal chromaticity stability, energy transfer and colour tunablity of Dy3+-Eu3+ doped BBZL glasses for high-powerW-LED applications. Ceram Int 49:5228–5238. https://doi.org/10.1016/j.ceramint.2022.10.040
Rajaramakrishna R, Nijapai P, Kidkhunthod P, Kim HJ, Kaewkhao J, Ruangtaweep Y (2020) Molecular dynamics simulation and luminescence properties of Eu3þ doped molybdenum gadolinium borate glasses for red emission. J Alloys Compd 813:151914. https://doi.org/10.1016/j.jallcom.2019.151914
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding for research.
Author information
Authors and Affiliations
Contributions
A. Ratep: Investigation, Writing—original draft, Methodology, Formal analysis. I. Kashif: Writing—review & editing, administration, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Ethics Approval
This paper meets the ethical standards of this journal.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kashif, I., Ratep, A. Various Color Light Emission from Single, Double, and Triple Eu3+/Tb3+/Tm3+ Doped Borate Glass Excited by UV Light. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03502-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03502-x