Abstract
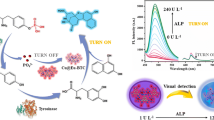
In this paper, a ratiometric fluorescence biosensor was introduced for alkaline phosphatase (ALP) detection based on 2-aminopurine (2-Amp) and thioflavin T (ThT)-G-quadruplex system. We designed a special DNA (5'-AGGGTTAGGGTTAGGGTTAGGGAAA/i2-Amp/AAAA-PO4-3', AP) modified with a phosphate moiety at the 3’-end, G-quadruplex at the 5’-end, and a fluorophore (2-Amp) in the middle. In the absence of ALP, the G-rich AP strand could be prone to fold into G-quadruplex structures in the presence of K+. Then, ThT combined with G-quandruplex, resulting in the enhancement of fluorescence emission peak at 485 nm. However, ALP-mediated hydrolysis of the 3’-phosphoryl end promoted the cleavage of AP by the exonuclease I (Exo I), releasing 2-Amp which displayed a strong fluorescence emission peak at 365 nm. Moreover, the quantitative fluorescence model (QFM) was derived for the analysis of the fluorescence measurements obtained by the proposed ratiometric fluorescent biosensor. With the aid of the advanced model, the proposed ratiometric fluorescent biosensor possessed satisfactory results for the detection of ALP in the human serum samples, with accuracy comparable to that of the reference method—the commercial ALP assay kit. Under the optimized experimental conditions, this method exhibited good selectivity and higher sensitivity, and the detection limit was found to be as low as 0.017 U/L. Therefore, it is reasonable to expect that the method had a great potential to detect ALP quantitatively in clinical diagnosis.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information file).
References
Zaher DM, El-Gamal MI, Omar HA, Aljareh SN, Al-Shamma SA, Ali AJ, Zaib S, Iqbal J (2020) Recent advances with alkaline phosphatase isoenzymes and their inhibitors. Arch Pharm 353:e2000011. https://doi.org/10.1002/ardp.202000011
Ma F, Liu WJ, Liang L, Tang B, Zhang C (2018) Sensitive detection of alkaline phosphatase by dephosphorylation-initiated transcription reaction-mediated dual signal amplification. Chem Commun 54:2413–2416
Wang K, Wang W, Zhang X, Jiang A, Yang Y, Zhu H (2021) Fluorescent probes for the detection of alkaline phosphatase in biological systems: recent advances and future prospects. TrAC-Trend Anal Chem 136:116189. https://doi.org/10.1016/j.trac.2021.116189
Makris K, Mousa C, Cavalier E (2023) Alkaline phosphatases: Biochemistry, functions, and measurement. Calcified Tissue Int 112:233–242. https://doi.org/10.1007/s00223-022-01048-x
Zhang Y, Guo L, Chen S, Yu Y, Wang J (2020) A portable photoacoustic device for facile and sensitive detection of serum alkaline phosphatase activity. Anal Chim Acta 1108:54–60. https://doi.org/10.1016/j.aca.2020.02.054
Wang Y, Yan Y, Liu X, Ma C (2021) An exonuclease I-aided turn-off fluorescent strategy for alkaline phosphatase assay based on terminal protection and copper nanoparticles. Biosensors 11:139. https://doi.org/10.3390/bios11050139
Gwynne L, Sedgwick AC, Gardiner JE, Williams GT, Kim G, Lowe JP, Maillard J, Jenkins ATA, Bull SD, Sessler JL, Yoon J, James TD (2019) Long wavelength TCF-based fluorescent probe for the detection of alkaline phosphatase in live cells. Front Chem 7:255. https://doi.org/10.3389/fchem.2019.00255
Kiran S, Khatik R, Schirhagl R (2019) Smart probe for simultaneous detection of copper ion, pyrophosphate, and alkaline phosphatase in vitro and in clinical samples. Anal Bioanal Chem 411:6475–6485. https://doi.org/10.1007/s00216-019-02027-2
Xu J, Jiang R, He H, Ma C, Tang Z (2021) Recent advances on G-quadruplex for biosensing, bioimaging and cancer therapy. TrAC-Trend Anal Chem 139:116257. https://doi.org/10.1016/j.trac.2021.116257
Balogun K, Lee M, Doyle K (2020) Comparison of Heat Fractionation and Gel Electrophoresis Methods for the Quantitative Determination of Alkaline Phosphatase Isoenzymes. Am J Clin Pathol 154: S8-S8. https://doi.org/10.1093/ajcp/aqaa137.014
Mahato K, Purohit B, Kumar A, Chandra P (2020) Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-dendroids and graphene oxide nanocomposite. Biosens Bioelectron 148:111815. https://doi.org/10.1016/j.bios.2019.111815
Gan X, Qiu F, Jiang B, Yuan R, Xiang Y (2021) Convenient and highly sensitive electrochemical biosensor for monitoring acid phosphatase activity. Sens Actuat B-Chem 332:129483. https://doi.org/10.1016/j.snb.2021.129483
Caviglia C, Carletto RP, De Roni S, Hassan YM, Hemanth S, Dufva M, Keller SS (2020) In situ electrochemical analysis of alkaline phosphatase activity in 3D cell cultures. Electrochim Acta 359:136951. https://doi.org/10.1016/j.electacta.2020.136951
Tian F, Zhou J, Ma J, Liu S, Jiao B, He Y (2019) MnO2 nanosheets as oxidase mimics for colorimetric detection of alkaline phosphatase activity. Microchim Acta 186:408–420. https://doi.org/10.1007/s00604-019-3519-1
Ni P, Chen C, Jiang Y, Zhang C, Wang B, Cao B, Li C, Lu Y (2019) Gold nanoclusters-based dual-channel assay for colorimetric and turn-on fluorescent sensing of alkaline phosphatase. Sens Actuat B-Chem 301:127080. https://doi.org/10.1016/j.snb.2019.127080
Shaban SM, Moon BS, Pyun DG, Kim D (2021) A colorimetric alkaline phosphatase biosensor based on p-aminophenol-mediated growth of silver nanoparticles. Colloid Surf B 205:111835. https://doi.org/10.1016/j.colsurfb.2021.111835
Li N, Chen H, Zhang M, Zha Y, Mu Z, Ma Y, Chen P (2020) A universal ultrasensitive platform for enzyme-linked immunoassay based on responsive surface-enhanced Raman scattering. Sens Actuat B-Chem 315:128135. https://doi.org/10.1016/j.snb.2020.128135
Liu H, Wei L, Hua J, Chen D, Meng H, Li Z, Xiao L (2020) Enzyme activity-modulated etching of gold nanobipyramids@MnO2 nanoparticles for ALP assay using surface-enhanced Raman spectroscopy. Nanoscale 12: 10390–10398. https://doi.org/10.1039/D0NR01837F
Zeng Y, Ren JQ, Wang SK, Mai J, Qu B, Zhang Y, Shen A, Hu J (2017) Rapid and reliable detection of alkaline phosphatase by a hot spots amplification strategy based on well-controlled assembly on single nanoparticle. ACS Appl Mater Interfaces 9:29547–29553. https://doi.org/10.1021/acsami.7b09336
He Y, Wang C, Zhao Q, Zhang Y, Chen A, Pang J, Fang Q, Cui Y, Jiao B (2017) Facile and sensitive fluorescence sensing of alkaline phosphatase activity using NMM/G-quadruplex. Talanta 172:171–175. https://doi.org/10.1016/j.talanta.2017.05.041
Cheng X, Chai Y, Xu J, Wang L, Wei F, Xu G, Sun Y, Hu Q, Cen Y (2020) Enzyme cascade reaction-based ratiometric fluorescence probe for visual monitoring the activity of alkaline phosphatase. Sens Actuat B-Chem 309:127765. https://doi.org/10.1016/j.snb.2020.127765
Lee S, Kim H, Kim HY, Park HG (2021) Target-induced transcription of a light-up RNA aptamer to construct a novel method for alkaline phosphatase assay. Chem Commun 57: 12341–12344. https://doi.10.1039/D1CC04787F
Zuo Q, Chen Y, Chen ZP, Yu R (2020) A novel ratiometric fluorescent sensing method based on MnO2 nanosheet for sensitive detection of alkaline phosphatase in serum. Talanta 209:120528. https://doi.org/10.1016/j.talanta.2019.120528
Zhou X, Khusbu FY, Chen H, Ma C (2020) A turn-on fluorescence assay of alkaline phosphatase activity based on an enzyme-triggered conformational switch of G-quadruplex. Talanta 208:120453. https://doi.org/10.1016/j.talanta.2019.120453
Pu L, Xia M, Sun P, Zhang Y (2021) Ratiometric fluorescence determination of alkaline phosphatase activity based on dual emission of bovine serum albumin-stabilized gold nanoclusters and the inner filter effect. Analyst 146: 943–948. https://doi.org/10.1039/D0AN01978J
Wang L, Chen S, Ma X, Wu Y, Tang Y, Hou S (2022) Fast and sensitive near-infrared ratiometric fluorescent probe with a self-immolative spacer for imaging of endogenous alkaline phosphatase activity in cells and in vivo. Talanta 249:123658. https://doi.org/10.1016/j.talanta.2022.123658
Amalraj A, Pavadai R, Perumal P (2021) Recyclable target metal-enhanced fluorometric naked eye aptasensor for the detection of Pb2+ and Ag+ ions based on the structural change of CaSnO3@PDANS-constrained GC-rich ssDNA. ACS Omega 6:30580–30597. https://doi.org/10.1021/acsomega.1c04319
Amalraj A, Perumal P (2022) Label-free DNAzyme for highly sensitive detection of multiple biomolecules in real samples through target-triggered catalytic cleavage reactions with auramine O’s discriminated fluorescence emission. Anal Bioanal Chem 414:4021–4037. https://doi.org/10.1007/s00216-022-04061-z
Amalraj A, Pavadai R, Subramanian S, Perumal P (2022) Fabrication of multi-functional CuO@ PDA-MoS2 mediated dual-functional fluorescence Aptamer for the detection of Hg2+ ions and chloramphenicol through desulfurization cleavage reaction and exonuclease I activity. Appl Surf Sci 602:154222. https://doi.org/10.1016/j.apsusc.2022.154222
Amalraj A, Chauhan NPS, Perumal P (2023) A dual-color fluorescent biosensing platform based on amine functionalized 3D copper prussian blue nanocubes and exonuclease I activity for simultaneous detection of kanamycin and streptomycin. New J Chem 47:3956–3969. https://doi.org/10.1039/d2nj06360c
He Y, Jiao B (2016) Simple and convenient G-quadruplex-based fluorescent assay of biotin-streptavidin interaction via terminal protection of small molecule-linked DNA. Microchim Acta 183:3303–3309. https://doi.org/10.1007/s00604-016-1980-7
Chen Y, Chen ZP, Yang J, Jin J, Zhang J, Yu R (2013) Quantitative fluorescence spectroscopy in turbid media: a practical solution to the problem of scattering and absorption. Anal Chem 85:2015–2020. https://doi.org/10.1021/ac302815e
Chen ZP, Morris J, Martin E (2006) Extracting chemical information from spectral data with multiplicative light scattering effects by optical path-length estimation and correction. Anal Chem 78:7674–7681. https://doi.org/10.1021/ac0610255
Brichacek AL, Brown CM (2019) Alkaline phosphatase: a potential biomarker for stroke and implications for treatment. Metab Brain Dis 34:3–19. https://doi.org/10.1007/s11011-018-0322-3
Qu F, Pei H, Kong R, Zhu S, Xia L (2017) Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta 65:136–142. https://doi.org/10.1016/j.talanta.2016.11.051
Funding
Open access funding is provided by the Hunan Provincial Education Department Scientific Research Outstanding Youth Project (No. 22B0579), the National Natural Science Foundation of China (No. 22204049), the Natural Science Foundation of Hunan Province (No. 2022JJ40042) and the Natural Science Foundation of Changsha (No. kq2202143).
Author information
Authors and Affiliations
Contributions
Yao Chen: conceptualization, methodology, data curation, software, writing-original draft. Jing-Jing Han: methodology, data curation. Bo-Wen Li: validation. Li-Bo Nie: formal analysis, writing-review & editing. Ying Tang: conceptualization, investigation. Tong Wang: funding acquisition, supervision.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Han, JJ., Li, BW. et al. A Ratiometric Fluorescence Biosensor for Detection of Alkaline Phosphatase Via an Advanced Chemometric Model. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03445-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03445-3