Abstract
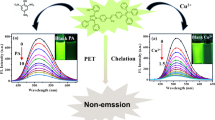
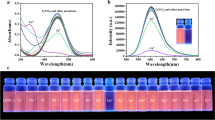
Herein, α-cyanostilbene-based luminogen with an electron donor-π-electron acceptor (D-π-A) architecture was formylated into the salicylaldehyde-analogue luminogen, followed by the Schiff base reaction with phenylamine, a red-emitting luminogen was elaborately designed and successfully synthesized in a high yield of 89%. Its well-defined structure was confirmed by FT-IR, MALDI-TOF-MS, HR-MS and 1H/13C NMR technologies. Based on the synergistic mechanisms of aggregation-induced emission (AIE), excited-state intramolecular proton transfer (ESIPT) and intramolecular charge transfer (ICT), it enjoyed a red-fluorescence emission at 627 nm in THF/water mixtures (fw = 95%) and was used as a probe. Moreover, the TLC-based test strips loaded with the probe not only exhibited the reversible fluorescence response to amine/acid vapor but also showed sensitive and selective fluorescence response towards Cu2+. Furthermore, the fluorescence titration experiment between the probe and Cu2+ in THF/water mixtures (fw = 95%, pH = 7.4) revealed that the detection limit was 1.18 × 10-7 M and the binding constant was 1.59 × 105. Job’s plot experiment and HR-MS analysis revealed the 2:1 binding stoichiometry of the probe with Cu2+. The method enabled real-time assessment for Cu2+ in real water samples. This study could offer insightful opinions on the development of long-wavelength emissive luminogens based on α-cyanostilbene.












Similar content being viewed by others
Data Availability
Data and material information is provided and will be shared on request.
References
Yamamoto N (2018) Mechanisms of aggregation-induced emission and photo/thermal E/Z isomerization of a cyanostilbene derivative: theoretical insights. J Phys Chem C 122:12434–12440. https://doi.org/10.1021/acs.jpcc.8b02147
Sun X, Liu Z, Wang Z, Huo M, Zhang HY, Liu Y (2022) Inclusion-activated reversible E/Z isomerization of a cyanostilbene derivative based on cucurbit[8]uril under 365 nm ultraviolet irradiation. J Org Chem 87:7658–7664. https://doi.org/10.1021/acs.joc.2c00185
Lin S, Gutierrez-Cuevas KG, Zhang X, Guo J, Li Q (2021) Fluorescent photochromic α-cyanodiarylethene molecular switches: an emerging and promising class of functional diarylethene. Adv Funct Mater 31:2007957. https://doi.org/10.1002/adfm.202007957
Ma X, Li P, Wang J, Yin M, Zhang Y (2022) Shape-dependent photomechanical motions of cyanostilbene-based molecular crystals. Cryst Growth Des 22:4133–4138. https://doi.org/10.1021/acs.cgd.2c00127
Chung JW, You Y, Huh HS, An BK, Yoon SJ, Kim SH, Lee SW, Park SY (2009) Shear- and UV-induced fluorescence switching in stilbenic π-dimer crystals powered by reversible [2 + 2] cycloaddition. J Am Chem Soc 131:8163–8172. https://doi.org/10.1021/ja900803d
Wu Y, Zhang S, Pei J, Chen XF (2020) Photochromic fluorescence switching in liquid crystalline polynorbornenes with α-cyanostilbene side-chains. J Mater Chem C 8:6461–6469. https://doi.org/10.1039/D0TC00069H
Jana P, Paramasivam M, Khandelwal S, Dutta A, Kanvah S (2020) Perturbing the AIEE activity of pyridine functionalized α-cyanostilbenes with donor substitutions: an experimental and DFT study. New J Chem 44:218–230. https://doi.org/10.1039/C9NJ03693H
Mahalingavelar P, Kanvah S (2022) α-Cyanostilbene: a multifunctional spectral engineering motif. Phys Chem Chem Phys 24:23049–23075. https://doi.org/10.1039/D2CP02686D
Niu G, Zheng X, Zhao Z, Zhang H, Wang J, He X, Chen Y, Shi X, Ma C, Kwok RTK, Lam JWY, Sung HHY, Williams ID, Wong KS, Wang P, Tang BZ (2019) Functionalized acrylonitriles with aggregation-induced emission: structure tuning by simple reaction-condition variation, efficient red emission, and two-photon bioimaging. J Am Chem Soc 141:15111–15120. https://doi.org/10.1021/jacs.9b06196
Luo J, Xie Z, Lam JWY, Cheng L, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D, Tang BZ (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun 18:1740–1741. https://doi.org/10.1039/B105159H
Yang Z, Chi Z, Mao Z, Zhang Y, Liu S, Zhao J, Aldred MP, Chi Z (2018) Recent advances in mechano-responsive luminescence of tetraphenylethylene derivatives with aggregation-induced emission properties. Mater Chem Front 2:861–890. https://doi.org/10.1039/C8QM00062J
Yu Z, Zhang Y, Wang C, Du X, Lu H, Wang Q (2021) Reversible mechanofluorochromic luminescence behaviors of 9, 10-distyrylanthracene- based compounds and their application in the rewritable papers technology. Dyes Pigm 190:109342. https://doi.org/10.1016/j.dyepig.2021.109342
Zhang JQ, Xu XY, Cao SQ, Zou YQ (2022) Structures and applications of NIR-II AIEgens containing benzobisthiadiazole derivatives. Eur J Org Chem 2022:e202201131. https://doi.org/10.1002/ejoc.202201131
Hsiao PY, Chu JH (2021) Novel bipyrazolo[1,5-a]pyridine luminogens with aggregation-induced emission enhancement properties. Chem Commun 57:12281–12284. https://doi.org/10.1039/D1CC05371J
Zhang H, Hu X, Zhu H, Shen L, Liu C, Zhang X, Gao X, Li L, Zhu YP, Li Z (2021) A solid-state fluorescence switch based on triphenylethene-functionalized dithienylethene with aggregation-induced emission. Front Chem 9:665880. https://doi.org/10.3389/fchem.2021.665880
Shen P, Zhuang Z, Zhao Z, Tang BZ (2018) AIEgens based on main group heterocycles. J Mater Chem C 6:11835–11852. https://doi.org/10.1039/C8TC02956C
Kim HJ, Nandajan PC, Gierschner J, Park SY (2018) Light-harvesting fluorescent supramolecular block copolymers based on cyanostilbene derivatives and cucurbit[8]urils in aqueous solution. Adv Funct Mater 28:1705141. https://doi.org/10.1002/adfm.201705141
Hang C, Wu HW, Zhu LL (2016) π-Conjugated cyanostilbene-based optoelectric functional materials. Chin Chem Lett 27:1155–1165. https://doi.org/10.1016/j.cclet.2016.04.003
Bala I, Kaur H, Maity M, Yadav RAK, De J, Gupta SP, Jou JH, Pandey UK, Pal SK (2022) Electroluminescent aggregation-induced emission-active discotic liquid crystals based on alkoxy cyanostilbene-functionalized benzenetricarboxamide with ambipolar charge transport. ACS Appl Electron Mater 4:1163–1174. https://doi.org/10.1021/acsaelm.1c01251
Wei P, Li Z, Zhang JX, Zhao Z, Xing H, Tu Y, Gong J, Cheung TS, Hu S, Sung HHY, Williams ID, Kwok RTK, Lam JWY, Tang BZ (2019) Molecular transmission: visible and rate-controllable photoreactivity and synergy of aggregation-induced emission and host–guest assembly. Chem Mater 31:1092–1100. https://doi.org/10.1021/acs.chemmater.8b04909
Mu B, Zhang Z, Hao X, Ma T, Tian W (2022) Positional isomerism-mediated copolymerization realizing the continuous luminescence color-tuning of liquid-crystalline polymers. Macromolecules 55:5332–5341. https://doi.org/10.1021/acs.macromol.2c00458
Shan X, Chi W, Jiang H, Luo Z, Qian C, Wu H, Zhao Y (2023) Monomer and excimer emission in a conformational and stacking-adaptable molecular system. Angew Chem Int Ed 62:e202215652. https://doi.org/10.1002/anie.202215652
Dhoun S, Kaur I, Kaur P, Singh K (2017) Propargylated cyanostilbene based chemodosimeter for Pd2+ with application in biological fluids. Dyes Pigm 143:361–367. https://doi.org/10.1016/j.dyepig.2017.04.054
Gong Y, Du C, Wang X, Guo H, Yang F (2021) First stable (Z)-configuration of cyanostilbene derivative: an effective “turn-on” fluorescent sensor for lysine in aqueous media. Microchem J 162:105866. https://doi.org/10.1016/j.microc.2020.105866
Bhaumik SK, Banerjee S (2021) Highly sensitive and ratiometric luminescence sensing of heparin through templated cyanostilbene assemblies. Analyst 146:2194–2202. https://doi.org/10.1039/D0AN01808B
Chen M, Ren Y, Liu H, Jiang Q, Zhang J, Zhu M (2021) A versatile aggregation-induced emission fluorescent probe for visible detection of pH. J Fluoresc 31:475–485. https://doi.org/10.1007/s10895-020-02669-x
Zhu M, Zhong M, Chen M, Huang S, Li Y, Cao F (2022) A π-conjugated α-cyanostilbene dimer emitting strongly red fluorescence with a large Stokes’ shift of ca. 300 nm and used as a probe for selective detection of Cu2+. Opt Mater 125:112059. https://doi.org/10.1016/j.optmat.2022.112059
Chen M, Huang W, Li Y, Chen Y, Ji D, Zhu M (2023) Preparation, AIE and ESIPT behaviour, controllable solid-state fluorescence and application of Co2+ probe based on α-cyanostilbene. Methods Appl Fluoresc 11:015002. https://doi.org/10.1088/2050-6120/aca378
Li B, Wang D, Lee MMS, Wang W, Tan Q, Zhao Z, Tang BZ, Huang X (2021) Fabrics attached with highly efficient aggregation-induced emission photosensitizer: toward self-antiviral personal protective equipment. ACS Nano 15:13857–13870. https://doi.org/10.1021/acsnano.1c06071
Gopinath A, Manivannan N, Mandal S, Mathivanan N, Nasar AS (2019) Substituent enhanced fluorescence properties of star α-cyanostilbenes and their application in bioimaging. J Mater Chem B 7:6010–6023. https://doi.org/10.1039/C9TB01452G
Sun Y, Sun P, Li Z, Qu L, Guo W (2022) Natural flavylium-inspired far-red to NIR-II dyes and their applications as fluorescent probes for biomedical sensing. Chem Soc Rev 51:7170–7205. https://doi.org/10.1039/D2CS00179A
Li H, Kim Y, Jung H, Hyun JY, Shin I (2022) Near-infrared (NIR) fluorescence-emitting small organic molecules for cancer imaging and therapy. Chem Soc Rev 51:8957–9008. https://doi.org/10.1039/D2CS00722C
Liu K, Jiang Z, Lalancette RA, Tang X, Jäkle F (2022) Near-infrared-absorbing B–N lewis pair-functionalized anthracenes: electronic structure tuning, conformational isomerism, and applications in photothermal cancer therapy. J Am Chem Soc 144:18908–18917. https://doi.org/10.1021/jacs.2c06538
Tian Y, Yin D, Cheng Q, Dang H, Teng C, Yan L (2022) Supramolecular J-aggregates of aza-BODIPY by steric and π–π nteractions for NIR-II phototheranostics. J Mater Chem B 10:1650–1662. https://doi.org/10.1039/D1TB02820K
Yang Y, Sun C, Wang S, Yan K, Zhao M, Wu B, Zhang F (2022) Counterion-paired bright heptamethine fluorophores with NIR-II excitation and emission enable multiplexed biomedical imaging. Angew Chem Int Ed 61:e202117436. https://doi.org/10.1002/anie.202117436
Mao W, Chi W, He X, Wang C, Wang X, Yang H, Liu X, Wu H (2022) Overcoming spectral dependence: a general strategy for developing far-red and near-infrared ultra-fluorogenic tetrazine bioorthogonal probes. Angew Chem Int Ed 61:e202117386. https://doi.org/10.1002/anie.202117386
Zhang T, Chen X, Yuan C, Pang X, Ping S, Liu Y, Han L, Sun J, Lam JWY, Liu Y, Wang J, Shi B, Tang BZ (2023) Near-infrared aggregation-induced emission luminogens for in vivo theranostics of alzheimer’s disease. Angew Chem Int Ed 62:e202211550. https://doi.org/10.1002/anie.202211550
Qiao W, Ma T, Wang S, Li L, Liu M, Jiang H, Wu Y, Zhu J, Li Z (2021) Designing squaraine dyes with bright deep-red aggregation-induced emission for specific and ratiometric fluorescent detection of hypochlorite. Adv Funct Mater 31:2105452. https://doi.org/10.1002/adfm.202105452
Sun L, Ouyang J, Ma Y, Zeng Z, Zeng C, Zeng F, Wu S (2021) An activatable probe with aggregation-induced emission for detecting and imaging herbal medicine induced liver injury with optoacoustic imaging and NIR-II fluorescence imaging. Adv Healthc Mater 10:2100867. https://doi.org/10.1002/adhm.202100867
Li L, Yuan G, Qi Q, Lv C, Liang J, Li H, Cao L, Zhang X, Wang S, Cheng Y, He H (2022) Synthesis of tetraphenylethene-based D–A conjugated molecules with near-infrared AIE features, and their application in photodynamic therapy. J Mater Chem B 10:3550–3559. https://doi.org/10.1039/D1TB02598H
Lv Z, Man Z, Cui H, Xu Z, Cao H, Li S, Liao Q, He Q, Zheng L, Fu H (2021) Red AIE luminogens with tunable organelle specific anchoring for live cell dynamic super resolution imaging. Adv Funct Mater 31:2009329. https://doi.org/10.1002/adfm.202009329
Bu L, Chen J, Wei X, Li X, Ågren H, Xie Y (2017) An AIE and ICT based NIR florescent probe for cysteine and homocysteine. Dyes Pigm 136:724–731. https://doi.org/10.1016/j.dyepig.2016.09.032
Huang Y, Mei J, Ma X (2019) A novel simple red emitter characterized with AIE plus intramolecular charge transfer effects and its application for thiol-containing amino acids detection. Dyes Pigm 165:499–507. https://doi.org/10.1016/j.dyepig.2019.02.053
Zhang F, Liu Y, Yang B, Wen G, Liu B (2020) Near-infrared AIEgens for lipid droplets imaging in corpus adiposum or trachea of Locusta migratoria and its application in photodynamic therapy. Sens Actuators B Chem 322:128589. https://doi.org/10.1016/j.snb.2020.128589
Li S, Zeng Y, Tang C, Wang F, Gu B, Tang S (2022) A red-emissive benzothiazole-based luminophore with ESIPT and AIE natures and its application for detecting and imaging hypochlorous acid. Spectrochim Acta A Mol Biomol Spectrosc 281:121601. https://doi.org/10.1016/j.saa.2022.121601
Guan P, Yang B, Liu B (2020) Fabricating a fluorescence resonance energy transfer system with AIE molecular for sensitive detection of Cu(II) ions. Spectrochim Acta A Mol Biomol Spectrosc 225:127604. https://doi.org/10.1016/j.saa.2019.117604
Gadiyaram S, Ghule VD, Ghosh A, Jose DA (2022) An ESIPT-based fluorescent probe for the detection of multiple analytes and a facile approach to discriminate between arsenate and pyrophosphate in water. Sens Diagn 1:1224–1235. https://doi.org/10.1039/D2SD00157H
Bhardwaj S, Maurya N, Singh AK (2018) Chromone based fluorescent organic nanoparticles for high-precision in-situ sensing of Cu2+ and CN‾ ions in 100% aqueous solutions. Sens Actuators B Chem 260:753–762. https://doi.org/10.1016/j.snb.2018.01.003
Maurya N, Singh AK (2017) Indirect approach for CN‾ detection via Cu2+ induced turn-off sensor: using novel AIEE fluorophore with logic gate and antimicrobial application. Dyes Pigm 147:484–490. https://doi.org/10.1016/j.dyepig.2017.08.046
Budri M, Naik G, Patil S, Kadolkar P, Gudasi K, Inamdar S (2020) An ESIPT blocked highly ICT based molecular probe to sense zn (II) ion through turn on optical response: experimental and theoretical studies. J Photochem Photobiol A Chem 390:112298. https://doi.org/10.1016/j.jphotochem.2019.112298
Synhaivska O, Bhattacharya S, Campioni S, Thompson D, Nirmalraj PN (2022) Single-particle resolution of copper-associated annular α-synuclein oligomers reveals potential therapeutic targets of neurodegeneration. ACS Chem Neurosci 13:1410–1421. https://doi.org/10.1021/acschemneuro.2c00021
Wang S, Sheng Z, Yang Z, Hu D, Long X, Feng G, Liu Y, Yuan Z, Zhang J, Zheng H, Zhang X (2019) Activatable small-molecule photoacoustic probes that cross the blood–brain barrier for visualization of copper(II) in mice with alzheimer’s disease. Angew Chem Int Ed 58:12415–12419. https://doi.org/10.1002/anie.201904047
Zhao J, Shi Q, Tian H, Li Y, Liu Y, Xu Z, Robert A, Liu Q, Meunier B (2021) TDMQ20, a specific copper chelator, reduces memory impairments in alzheimer’s disease mouse models. ACS Chem Neurosci 12:140–149. https://doi.org/10.1021/acschemneuro.0c00621
Chen M, Zhong M, Huang S, Chen Y, Cao F, Hu H, Huang W, Ji D, Zhu M (2023) α-Cyanostilbene-based sensor with “AIE and ESIPT” features emitting long-wavelength intense red-fluorescence for highly selective and sensitive detection of Cu2+. Inorg Chem Commun 152:110640. https://doi.org/10.1016/j.inoche.2023.110640
Acknowledgements
We are grateful for the financial support from the Undergraduate Innovation Program in Neijiang Normal University (No. X2022054 and No. X2022269).
Funding
This work was financially supported by the Undergraduate Innovation Program in Neijiang Normal University (No. X2022054 and No. X2022269).
Author information
Authors and Affiliations
Contributions
Meihui Chen: Conceptualization, Investigation, Writing - original draft. Yongchun Chen: Investigation. Min Zhong: Investigation. Donghong Xie: Investigation. Chuan Wang: Investigation. Xiaorui Ren: Investigation. Shizhou Huang: Investigation. Jia Xu: Investigation. Mingguang Zhu: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
Corresponding author
Ethics declarations
Ethics Approval
There is no ethic approval required for this research work.
Consent to Participate
Not applicable.
Consent for Publication
All authors agree for the publication.
Conflicts of Interest
No potential conflict of interest is reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Chen, Y., Zhong, M. et al. The Synergistic Mechanisms of AIE, ESIPT and ICT in the α-cyanostilbene-based Derivative: A Red-fluorescence Probe With a Large Stokes’ Shift for Copper (II) Ion Determination and Reversible Response to Amine/acid Vapor. J Fluoresc 34, 1075–1090 (2024). https://doi.org/10.1007/s10895-023-03341-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03341-w