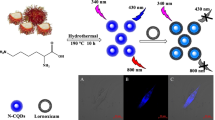
Abstract
The present work highlights the sustainable approach for the transformation of plastic waste into fluorescent carbon dots (CDs) through carbonization and then they were functionalized with L-cysteine and o-phenylenediamine. CDs which were characterized by different analytical techniques such as X-ray diffraction (XRD), thermogravimetric analysis (TGA), Scanning electron microscopy (SEM), and transmission electron microscopy (TEM) are employed to recognize Cu2+, Fe2+, and Hg2+ ions. The results show that the fluorescence emission was considerably quenched, and it is consistent with the interference and Jobs plots. The detection limit was found to be 0.35µM for Cu(II), 1.38 µM for Hg(II), and 0.51µM Fe(III). The interaction of CDs with metal ions enhances the fluorescence intensity detecting histamine successfully. It shows that plastic waste-based CDs can be applied clinically to detect toxic metals and biomolecules. Moreover, the system was employed to develop the cellular images using Saccharomyces cerevisiae cells with the support of a confocal microscope. Furthermore, theoretical studies were performed for the naphthalene layer (AR) as a model for C-dots, then optimized its structure and analyzed by using the molecular orbital. The obtained TD-DFT spectra coincided with experimental spectra for CDs/M2+/histamine systems.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymeric materials like polyethylene terephthalate (PET) are being used as industrial containers for the preservation of medicines, food, and soft drinks because of excellent physical and chemical properties such as high thermal stability, transparency, flexibility design, waterproof, good insulating character, low electrical conductivity, and inertness towards acids, sunlight, and microorganisms [1, 2]. It has been popular because of its low cost, easy to handle, lightweight, high strength, and long durability [3,4,5]. However, the accumulation of huge quantities of plastic waste in the environment is a serious issue [6] due to its non-degradable nature, contributing significantly to health and ecological imbalance [7,8,9]. Polymeric plastics contain a long carbon chain of about 62.6–92.2% [10] such as polyethylene (-C2H4)n, polypropylene (-C3H6)n, polystyrene (-C8H8)n, polyvinyl chloride (-C2H3Cl)n, etc., [11, 12] and chemical recycling, or converting to gaseous, liquid, carbon-enriched materials are being considered [13, 14]. The incineration of PET is strictly prohibited as it consumes a lot of O2/air (14 m3 kg− 1), causing toxic- and greenhouse effects [15, 16]. The biodegradation of plastics by mealworms [17, 18], and the transformation of plastic- waste into adsorbents are also considered [19].
The conversion of PET waste to carbon-based nanomaterials (carbon nanotubes, graphene, and Carbon Dots (CDs)) turned out to be interesting [2, 20,21,22,23,24] as they exhibit good optical properties [25]. CDs (size, < 10 nm) can be used as bio-imaging agents, photo-catalyst, sensors, and solar cells [24, 26,27,28,29]. It has been found that CDs are somewhat superior to metal-based particles with respect to biocompatibility, and environmental friendliness [30, 31]. The covalent carbon skeleton also enhances the stability of CDs [32, 33] as it contains carbon, oxygen, and nitrogen with a mixture of sp2/sp3 carbon lattices. If CDs were functionalized properly, water-soluble CDs can be obtained [16, 31, 34]. Chemo-sensors have been widely applied for the detection of multiple heavy metals and their sensitivity can be improved by increasing the large surface/volume ratio after considering a high degree of functionalization [35, 36].
The formation of CDs from polymer wastes is attractive and can attribute to the different degrees of carbonization [21]; however, these types of studies are limited in the literature. So, the present work deals with the eco-friendly CDs-based chemo-sensor for the detection of metal ions, emerging as an alternative technique [37,38,39]. Interestingly, the generation of CDs from plastic waste is considered to be an eco-friendly method as the CDs are formed from biomass carbon [40], peanut shells [41], orange juice [42], Jinhua bergamot [43], and lotus root [44]. It is known that CDs have been used in different applications such as in the interaction with different heavy metals, namely Cu2+ ions [45,46,47]; in the logic gate operation [48]; in the detection of ascorbic acid, phosphatase [49], glyphosate [50] or glutathione [46]; also in the monitoring of pesticide [51]; in the development of cell image/Cu2+ [37]. In addition, polyamine-functionalized carbon quantum dots also have been used for the determination of copper ions [52]. Furthermore, CDs have been doped with other materials such as N-co-doped CDs [53], N, S-CDs [54], S-CDs [55], CDs/Ag [56], Hg2+ [57] to improve their efficiency for the recognition of Hg2+ [58,59,60].
The presence of toxic metals such as copper in environmental samples affects drastically biological functions [61], causing oxidative stress that produces disorders related to diseases like Menkes, Wilson’s, Parkinson’s, and Alzheimer’s. Controlling copper content in water is challenging although there are several methods like atomic absorption spectrometry (AAS) [62,63,64], inductively coupled plasma atomic emission spectroscopy (ICP-MS) [65,66,67], GC-MS, LC-MS or electrochemical analysis [68] are being employed to determine the concentration of metal ions. However, these techniques are usually time-consuming due to the complicated sample preparation, complex sample processing, and expensive instrumentation. In the present work, we report the plastic waste-based fluorescent carbon dots (FCDs) to recognize Fe3+, Cu2+, and Hg2+ ions along with histamine, which has been observed in spoiled food and used often as an indicator for food safety; therefore, the development of a rapid and sensitive method for the detection of histamine is essential. Thus, the present sequential system has been applied to develop the cellular images using Saccharomyces cerevisiae ATCC 9763 cells with the help of confocal microscope. The CDs were characterized appropriately by different analytical techniques such as XRD, FT-IR, TGA, SEM, and TEM. The intensity of fluorescence was quenched for the metal ions while for histamine, it was enhanced. Furthermore, the theoretical studies were performed for a single nanographene layer (AR) which has been considered a model for C-dots and studied the interaction of CDs with metal ions and histamine. To the best of our knowledge, there is no report on the performance of the functionalized CDs derived from plastic wastes for the detection of metal ions performing as a logic gate for the recognition of histamine. DFT was used to probe the function of CDs/Mn+/histamine in the absorption spectra.
Materials and Methods
Materials
Chemicals and solvents (Sigma Aldrich) were purchased and used without further purification. Plastic bottles were collected from the waste cabbages and were recycled/ transformed into CDs.
Synthesis of CDs
PET bottles were sized into small pieces (~ 1.0 × 1.0 cm2) and placed in a ceramic crucible to heat at 400 °C for 2 h in air. A dark brown product formed was crushed to a solid (PET-C). The obtained product (0.25 g) was mixed with L-cysteine (0.5 g) and o-phenylenediamine (0.25 g) and dissolved in de-ionized water (30 mL). The resulting mixture was transferred to a Teflon-contained autoclave and heated at 200 °C for 8 h. A brownish-black residue obtained was centrifugated (9000 RPM, for 20 min), filtered, and the filtrate was dried at 80 °C for 4 h to obtain the CDs, (Scheme 1).
For CDs samples, the XRD was performed on a Bruker D8 Advance Davinci, using Ni filter for Cu Kα radiation (λ = 1.541Å), and the diffraction angle at 2θ was in the range of 20° to 80° operating at a voltage of 40 kV, 30 mA. The crystallite size of CDs was calculated by using Scherer’s formula. The morphology and size of the CDs were analyzed by scanning electron microscopy (SEM, JEOL JSM-5900-LV). The nature of CDs was studied by transmittance electron microscopy (TEM, JEOL JEM-2010, resolution: 3.0 nm HV), equipped with an EDX detector, fully automated on all 5 axes. Infrared spectra (4000 cm− 1 to 600 cm− 1, Perkin-Elmer Spectrum 400 FTIR/FIR) were recorded for CDS to determine the functional groups like -C = O, -OH, and -NH2. The thermal stability of samples was studied by thermogravimetric analysis (TGA METTLER TOLEDO DSC1) in alumina cuvettes; airflow: 50 mL/min for each sample (10 ± 1 mg) with a precision of ± 0.02 °C ± 0.2 °C; heating range: 50 to 900 °C. For CDs samples, the fluorescence properties were studied on F-96 Pro and the absorption spectra were measured on Perkin-Elmer Lambda 25.
Recognition Studies
First, metal nitrate solution (10 mM) of different cations such as Al3+, Ca2+, Cd2+, Co3+, Cu2+, Fe3+, Hg2+, Ni2+, K+, Mg2+, Ba2+, Na+ and Zn2+ was prepared. The metal binding studies were performed by adding aliquots of stock solutions of the respective metal ions to the CDs solution (0.016 ppm), which was buffered with an aqueous solution of HEPES (20 mM at pH = 7.0). The final concentration of [M]final was 20 µM. The intensity of fluorescence emission of the sample was recorded at 387 nm. The titration of CDs with metal ions was also carried out by adding aliquots of stock solutions (analytes: Fe3+, Cu2+, Hg2+) to CDs (0.016 ppm) which were buffered as indicated above. The whole mixture solution was allowed to equilibrate for 2 min at room temperature and then recorded the emission spectra at 310 nm using a 10 mm quartz cuvette. For each successive addition of metal ions (Cu2+, Hg2+, or Fe3+ (0 mM to 0.62 mM) to the CD solution (0.016 ppm), the decrease of the intensity was analyzed. The stoichiometry of the receptor CDs with the metal ions (especially with Cu2+) was determined by Job’s plot. The different proportions of CDs: Cu2+ was prepared as follows: 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1, and then measured their fluorescence intensities which were then plotted against the concentration as (I-Io)·X vs. X (X=[CDs ]/[CDs ]+[M]).
The limit of detection (LOD) for CDs to Cu2+, Hg2+, or Fe3+ was determined from the plot (fluorescence intensity vs. the concentration of the metal ion). The fluorescence quantum yield was also calculated using the equation:
Фfs = quantum yields of sample; Фfr = quantum yields of reference sample; As = absorbance of sample and Ar = absorbance of the reference; Ls and Lr = lengths of the absorption cells; ns and nr = refractive indices of the sample and reference solutions, respectively [69]. PL quantum yield (Фfr) was measured using quinine sulfate in sulfuric acid solution (0.05 M) as a reference (literature quantum yield was 0.54 at 310 nm nr = 1.33) as a standard for carbon dots. In order to minimize re-absorption effects, the fluorescence cuvette was kept under 0.05 at the excitation wavelength [70].
Cellular Imaging
The potential for cellular imaging of the CDs was tested by using Saccharomyces cerevisiae ATCC 9763 cells. Yeast cells were suspended in 20 mL of distilled water to which CDs were dispersed (0.016 ppm). Yeast cells were smeared on a microscope slide and treated for 10 min with CDs dispersion (25 µL of 0.016 ppm). Finally, the samples were observed at an epi-fluorescent microscope (Carl Zeiss AXIO Scope A1).
Results and Discussion
Characterization
FT-IR, XRD, and TGA: CDs are derived from the plastic contain mostly C, H components and do not have any hetero atoms (functional groups); however, CDs (from o-phenylenediamine and L-cysteine) might have -NH2, -OH, -C-C- and -C-H groups. Therefore, we have used IR technique to observe the signals corresponding to -C-C-, -C-H, -NH2 and -OH bonds presenting in the CDs. In the FT-IR spectra (Fig. 1a), it has been observed the signals corresponding to O–H and N–H (3349 cm–1), C–H (2890 cm–1), C–O (1623 cm–1), C = C (1575 cm–1), C – N (1416 cm–1 ) and C – O (1214 cm–1) [71]. The peaks of S-H (2633 cm –1), C = S (1311 cm–1), C – O (1003 cm–1), C = C (1597 cm –1), and C = O (1705 cm–1). The results show that the surface of CDs was enriched with hydroxyl, carbonyl, amino, and thiol groups that support the water solubility of the sample. XRD was performed for CDs and noticed a broad peak at 26° with the prominent 2θ value, corresponding to the (002), and the size of the particle was D = 0.39 nm, corresponding to abounding sp3 defects of carbon-based materials. The appearance of the characteristic peak has coincided with those reported in JCPDS 41-1487 (graphite) [72] (Fig. 1b). This means that the interlayer spacing (around 0.39 nm) is approximately agreed with that of the graphite 002 crystal plane (0.34 nm). CDs have a wider interplanar spacing, which may be caused by the doping of heteroatoms (such as N and S) which generally increase the repulsive force between the layer of the molecules. The electronegativity of N is larger than that of C, but the atomic radius is smaller than C. The crystalline CDs (Fig. 1b) were found to be triclinic types [16]. In contrast, the plastic waste that was carbonized (PET-C) shows a sharp diffraction peak at approximately 21° with a center of 2θ, as can be seen from the XRD peak shape that the PET-C is presented a crystalline state. The average grain size (D) was calculated by Scherrer’s formula [73].
where λ is 0.15406 nm, θ is the Bragg angle, and B is the full width at half maximum.
The thermal performance of the sample was studied by the TGA technique (Fig. 1c) and the results show that CDs are significantly stable up to 100 °C. In the plot, two prominent bending of weight loss caused by the moisture was observed and it is also contributed by the pyrolysis of the functional groups from the CDs, and it is consistent with the weight loss around 250 and 575 °C [74]. This means that the weight loss was observed at stepping temperature after 100 °C as there is water evaporation and the burning of both carbon surface and carbon core structures occurred around 400 °C [75].
The SEM was recorded for CDs and PET-C (Fig. 2Ia-b) and determined the morphology of particles. The CDs present a regular morphology in spherical and porous shapes, in addition to uniformity. Mostly, CDs are agglomerated in the form of clusters, showing that the size of CDs is much smaller than that of the PET-C which is presented in a porous/irregular shape with little uniformity (there is no agglomeration). The dimension of the CD is one of the most important properties because this allows a greater area of contact with the contaminants and therefore their interaction, the PET-C not having a nanometric size prevents it from being used as a chemo sensor as well as its low affinity with other elements and particles. EDS was used to estimate the elemental composition for CDs: C (77.5%), O (16.5%), S (5.5%), and N (0.6); for PET-C: C (78.1%), O (20.5%) and S (1.4%), which verifies the presence of N and S derived from the addition of L-cysteine and o-phenylenediamine to the synthesis to improve its characteristics. For both cases, Carbon is the element with the highest mass percentage present in nanomaterials.
Transmission electron microscopy (TEM) imaging as described provides information about the structure, phases, and orientations of the sample, whether it is amorphous or crystalline. TEM was used to characterize the microstructures of the CDs. The material showed uniform dispersion and spherical particles between 3 and 10 nm, with average diameters of approximately 4.91 nm (Fig. 2IIa-b). TEM images showed that the CDs had an average lattice spacing of approximately 0.213 nm well interplanar spacing; when the molecules are located in adjacent parallel planes and each of these planes has a designated Miller index, these indices are made up of three numbers which indicate the coordinates of a vector in three dimensions (x, y, z), corresponding to the (0 0 2) planes of graphite, which when compared to the clear, strong, broad X-ray diffraction (XRD) peak centered at around 26° indicated that the CDs had a graphitic structure, indicating the good crystallinity of CDs.
Metal Binding Analysis
UV-Vis absorption and fluorescence emission (PL) spectra were performed for the CDs in an aqueous solution, presenting pale yellow under visible light, and a bright blue color under fluorescence illumination (310 nm). The results show an absorption peak at 280 nm (Suppl. Mat. Fig. S1.), corresponding to n-π* and π-π* transitions from C = C and C = O, presenting in CDs [76], and determined minimum bandgap energy [77, 78]. Fluorescence emission was observed after excitation at 310 nm with high intensity at 392 nm, showing that the conjugated π-electrons are cross-linked with different functional groups through which it has enhanced the fluorescence emission [76]. Table 1 enlists the different CDs originated from different carbon sources and their photoluminescence properties and kind of detection ion. The quantum yield was measured to be 31.81% and it was estimated using quinine sulfate as a theoretical standard [79]. The result is excellent as compared to the carbon dots prepared from other polymers (Table 2). High quantum yields resulted for our FCDs were due to the high doping of nitrogen and sulfur into the CDs, leading to the formation of favorable emissive states.
The metal binding analysis of CDs was explored by adding aliquots of stock solutions of the respective cation as nitrate salt (Al3+, Ca2+, Cd2+, Co3+, Cu2+, Fe3+, Hg2+, Ni2+, K+, Mg2+, Ba2+, Na+, and Zn2+), final concentration of [M]final was 20 µM, after buffering with aqueous HEPES (20 mM at pH = 7.0) of CDs (0.016 ppm) (Suppl. Mat. Fig. S2). For the mixture solution, the fluorescence intensity measured at 387 nm was plotted against the respective cations (Na+, K+, Mg2+, Ca2+, Ba2+, Al3+, Co3+, and Zn2+). Notably, the addition of hard paramagnetic metal ions such as Ni2+ has resulted in a modest decrease in emission intensity, but it was still significantly lower than that observed for Fe3+ (I0/IF = 8.12) as well for Cu2+ (I0/IF = 10.30). On the other hand, the addition of a soft diamagnetic metal ion, namely, Cd2+ gave a small quenching in the intensity, and yet it was considerably lower than that detected for Hg2+ (I0/IF = 7.68). In general, the presence of -NH2 and -SH groups in CDs increases their affinity toward soft ions such as Cu and Hg as compared to hard metal ions. It means that the existence of paramagnetic centers favors the quenching of CDs as compared to the diamagnetic metal ions. After analyzing the results, it was noticed a significant quenching in the fluorescence intensity for Cu2+, Hg2+, and Fe3+, attributing to paramagnetic or heavy atoms and it influences the photo-induced electron transfer (PET) (Scheme 2).
Furthermore, the spectral titration was performed for Cu2+ and Hg2+ with CDs (0.016 ppm) in an aqueous solution and it was also seen a quenching of emission with the increasing concentration of Cu2+ or Hg2+ (Suppl. Mat. Fig. S2). The intensity of the fluorescent emission was measured for each successive addition of Cu2+ (10 mM), decreasing considerably for M = Cu2+, Hg2+, and Fe3+ (0 mM to 0.62 mM). The plot was drawn for the concentration with the fluorescence intensity giving good linearity.
LOD is found to be 0.35 µM (R2 = 0.997) for Cu2+; 1.058 µM (R2 = 0.989) for Hg2+ and 0.510 µM (R2 = 0.993) for Fe3+. Using the 3σ method, the limit of detection (LOD) for CDs was determined as follows:
Job’s Plot
The stoichiometric ratio of FCDs with M = Cu2+, Hg2+, Fe3+ was analyzed through a Jobs plot (Fig. 3). A solution of metal ion solution and CDs (0.016 ppm) was prepared and maintained the stoichiometry concentration (0 ppm to 100 ppm) for metal ions and 100 ppm to 0 ppm for CDs and the volume of solution in the cell was 2.5 mL. The ratio for CDs (80 ppm) with Cu2+ (20 ppm) was 0.8:0.2; for CDs (76 ppm) with Hg2+(24 ppm) was 0.76:0.24; and for CDs (60 ppm) with Fe3+ (40 ppm) was as 0.6:0.4.
Turn-on Histamine Detection: Turn-on Histamine Detection
As a first step, the relative selectivity of the CDs-Mn+(Cu2+, Fe3+, Hg2+) towards diamines was analyzed using histamine, ethylendiamine, cadaverine, putrescine, spermine, spermidine, dimethyl-1,3-propanediamine and dimethylethylenediamine ([Diamine]final = 0.01mM). The diamines were added to a buffered aqueous solution of CDs-Mn+(0.6437 ppm) and measured the emission intensity. The results show an enhancement in the intensity for the CDs-Mn+ as shown in Suppl. Mat. Figure 8. The fluorescence intensity was exceptionally low for the CDs-Mn+ with the addition of diamine such as cadaverine, putrescine, spermine, spermidine and dimethyl-1,3-propanediamine. For the addition of ethylendiamine and dimethylethylenediamine has exhibited a modest enhancement of the emission intensity, but it was still significantly lower than that observed for histamine. So, the fluorescence detection of histamine was performed by using CDs having metal ions (Cu2+, Fe3+ or Hg2+), showing a turn-on fluorescence with respect to the concentration of histamine (Scheme 2, Fig. 4a-c). It is established a best linear fit for histamine (0-1.23mM) (see Job’s plot, Table S1). The limit of histamine detection for each metal ion was: 0.193 µM for Cu2+, 0.36 µM for Hg2+, and 2.76 µM for Fe3+. In the presence of histamine, the fluorescent CDs can be recovered because of the strong interaction between metal ions (Cu2+, Fe3+ or Hg2+) and histamine. In this system (Mn+-CDs), the functional groups on the surface of CDs (carboxylates, amines, thiols, hydroxyls) serve as the recognition sites and it supports to the binding of amino group with imidazole from histamine in order to form a coordination complex that effectively restores the fluorescence of CDs. The coordination complex of histamine with Cu2+, Fe3+ or Hg2+ is thermodynamically stable due to the chelate effect generated from histamine which acts a chelating ligand, showing a high affinity for a metal ion than the analogous monodentate ligands.
The Job’s plot for histamine with CDs having Cu2+, Hg2+, or Fe3+ was determined (Fig. 3b), showing that with the increased concentration of histamine (0-150 µM), the FL intensity for CDs was increased (see Table S2).
Logic Gate Systems for CDs
Logic gates for CDs are important for detecting various analytes (cations, anions, amino acids, pesticides, antioxidants, etc.), and generally most of the logic gates are fundamentally based on fluorescence spectroscopy because of their sensitiveness. After analyzing the present results, it was found that there exists a logic gate system in the recognition of histamine in the presence of metal ions (Cu2+, Hg2+, Fe3+). This means, the enhancement of fluorescence intensity for histamine with CDs was seen only if the metal ions are presented in sequential order; however, the intensity was low or absent if we mixed other combinations of CDs with histamine. First, an AND logic gate was performed through the binding of CD with metal ions [A = 1, B = 0], turning off the fluorescence was seen at 450 nm. Nevertheless, the fluorescence intensity was increased if (other AND logic gate) in the order of CD/Cu2+, CD/Hg2+, CD/Fe3+ as an input (in the presence of histamine). This indicates that CDs first recognize the metal ions, and then detect histamine (Scheme 3).
The enhancement of fluorescence intensity has occurred only when all inputs [A = 1, B = 1, C = 1] are given, as it functions as a three inputs AND logic gate system. But, for other combinations with negative inputs [A = 0, B = 0, or C = 0], no fluorescence was exhibited (Table 3).
Cellular Imaging
Yeast Imaging with CDs
Saccharomyces cerevisiae ATCC 9763 cells were observed by epi-fluorescent microscope and recorded the microscopic images of yeast cells with CDs (0.016 ppm) (Fig. 5, left column; clear camp microscopic images, column center; phase-contrast microscopic images, and column right; microscopic images with a fluorescence filter of FITC). Row A corresponds to the yeast control sample without CDs, and B row represents the yeast cells with CDs, which demonstrated the suitability of CDs serving as a potential alternative fluorescence probe for the cell imaging.
Yeast Cell Imaging with Cu(II)-CDs and Histamine
The image of yeast cells were also observed in the presence of Cu(II)-CDs and the fluorescence intensity corresponding to CDs is quenched because of copper(II) as it is a paramagnetic ion (Fig. 5, C row). In the yeast cell imaging with Cu(II)-CDs and histamine, the cell images were observed in the presence of Cu(II)-CDs having histamine. It can be seen that in the presence of histamine, the fluorescent CDs can be recovered due to the existence of a strong interaction between Cu(II) and histamine. For Cu(II)-CDs, the functional groups (-COOH, -NH2, -SH) on the surface of CDs perform as active sites forming a metal complex through amino group and imidazole from histamine; thus, it regenerates effectively the fluorescence intensity (see Fig. 5, D row). The E Row corresponds to CDs with histamine, showing that histamine does not modify the fluorescence from CDs.
Microscope images: A row yeast cells, B row cells with CDs, C row cells with Cu(II)-CDs, D row cells with Cu(II)-CDs-Histamine, and E row cells with histamine. (Left column) clear camp microscopic images, (Center column) phase-contrast microscopic images, and (Right column) microscopic images with a fluorescence filter of FITC
Theoretical Studies
Theoretical Model
Structural optimization of naphthalene (AR) as a model for Carbon dots was studied by the DFT using Gaussian 09 having B3LYP and analyzed the influence of different functional groups (-COOH, OH, NH2) in the electronic and geometrical properties (Fig. 6(I)a and 6(I)b)0. The interaction of AR (other substituents such as -COOH, -OH, -NH2, and -SH) with metal ions (Fe3+, Cu2+, and Hg2+) was investigated as indicated previously [101], and derived TD-DFT spectra for the above compounds [102,103,104] at the DGDZVP basis set [101] .
I). Optimized molecular structure: (a) AR represents C-dots and (b) C-dots with functional groups; AR-COOH, AR-NH2, AR-OH, and AR–SH. II). Optimized geometries for C-dots which interacted with the metal ions; i): AR-Fe3+, AR-HCOO–Fe3+, AR-HO–Fe3+, AR-C–O–Fe3+ ii): AR-Cu2+, AR−HCOO–Cu2+, AR−HN–Cu2+, and iii): AR−Hg2+, AR−SH–Hg2+, III) Optimized geometry of AR with metal ions (Fe3+, Cu2+, and Hg2+) + histamine: a)AR-COOH-Fe-histamine; b)AR-OH-Fe-histamine, c) AR-COOH-Cu-histamine, d) AR-NH2-Cu-histamine, and e) AR-SH-Hg-histamine.
In the interaction of metal ions with molecule AR forms bond Fe-C (AR) (1.937 Å), Fe-O(COOH) (1.893 Å), Fe-O(OH) (2.068 Å), Fe-H(OH) (1.600 Å) and Fe = O (1.820 Å). Similarly, for Cu –C (AR) (1.940 Å), C-O(COOH) (1.937 Å), Cu-N(NH2) (1.933 Å) and N-C (AR) (1.314 Å) and for Hg-C(A) (2.364 Å), Hg-S (2.836 Å) and H – C(AR) (2.437 Å) were obtained (Fig. 5(II)). The results show that the bond length of carbon with Fe3+ (1.937 Å) or with Cu2+ (1.940 Å) is relatively shorter than with Hg2+ (2.364 Å). In particular, the metal ions coordination with other functional groups attached to ligand (AR) studied was showing the formation of Cu2+-O(COOH) (1.973 Å), Cu2+-NH2 (1.933 Å) and Fe3+-O(COOH) (1.893 Å), (Fe3+-O(OH) (2.068 Å) and Hg2+-SH (2.836 Å). This is consistent with the molecular orbital studies where the overlapping of the orbital of AR with that of Fe3+ and Cu2+ ions was noticed (Supp. Mat. Fig. S9). In general, in the study, a significant change in the bond distance of M-AR (M = Fe3+, Cu2+, Hg2+) was observed if AR possesses different functional groups (AR-COOH, OH, NH2, SH) (Table S3). For example, the presence of a strong bond was seen for Fe3+ and Cu2+ with AR as compared to Hg2+, for which, the bond length was somewhat longer. Thus, AR with functional groups is suitable for the detection of Fe3+ and Cu2+ (Supp Mat. Fig. S10, Table S5).
Recently, the optical and electronic properties of the quantum dots based on graphene were reported [105]. Accordingly, we have proposed an adequate model that represents C-dots after considering a single nano-graphene layer; however, in a realistic model, several layers could be aggregated (Fig. 6I, II, III). The TD DFT absorption spectrum (Fig. 7) calculated for the above structures somewhat coincided with that observed for the C-dots which were prepared by hydrothermal method [106,107,108,109,110], in particular, the absorption spectral peaks (200–400 nm) have coincided approximately with the experimental values [108, 110]. The C-dots structures consist of amorphous carbon having functional groups during the carbonization of organic molecules [109].
TD-DFT absorption spectra for C-dots: a without functional groups and AR-COOH; b with functional groups on the surface of C-dots and AR-OH), c without functional groups and AR-NH2; d without functional groups and AR-HS; and e for AR as C-dots with M3+/2+, M = Fe3+, Cu2+, and Hg2+) and (f) AR, COOH, Fe3 + and histamine group
In the spectra, the peak appeared approximately around 200–247 nm for AR having functional groups (Fig. 7) [111], representing two aromatic rings having several carbon atoms [112]; it means that the peak representing C-dots was observed for the π-π* transition [106, 109]. Thus, the chain of AR carbon rings is considered a model for C-dots (see Supp. Mat. Fig. S9). The electronic and aromatic properties of nanographene are highly dependent on the aromaticity of the rings [113, 114] and the particular peak can be shifted if the aromaticity of the Hückel rule is satisfied for aromatic rings attached with different functional groups. Several reports [109, 110, 115,116,117] describe carbon dots possess different functional groups on their surface; thus we have proposed the selected structures represent the carbon dots’ surface.
The influence of the functional groups in the absorption spectra was analyzed (Fig. 6a-d, and see Supp. Mat. Table S4), observing that the peak associated with π-π* transitions underwent a redshift. The visible absorption band is associated with the metal ion d-d electronic transition [106, 109]. The interaction of AR as C-dots with iron, copper, and mercury was studied as AR -Fe3+, AR-Cu2+, and AR -Hg2+ (Fig. 7e). The results show clearly the presence of three absorption peaks around 435 nm for AR -Fe3+, 431 nm for AR-Cu2+, and 329 nm for AR -Hg2+. After an analysis of their DOS (Fig. S7 (ii)), the transitions between d-occupied states and s-unoccupied states occurred, agreeing with a pure quadrupole transition selections rule l = ± 2, where l is the angular momentum quantum number [53]; as a result, these peaks 244, 266, and 214 nm were obtained. Similarly, the transitions between occupied bands (d and s) to unoccupied bands (p) can occur for the l = ± 1 dipole transition selection rule, but the peak can appear at lower wavelengths [50]. In the present work, the AR molecule as C-dots with different functional groups (COOH, NH2, and HS) can interact with metal ions followed by histamine molecule; thus, in the structural calculation, a stable configuration of these adducts were obtained.
The adsorption energies and the bond distances (Mat. Supp, Table S5) for the interaction of CDs with Fe3+, Cu2+, and Hg2+ were obtained and they show that a stable bonding was seen for AR-COOH-Fe-Nhistamine and AR-COOH-Cu-Nhistamine. However, for AR -SH-Hg, that interaction has not been stabilized adequately; but it has enough energy to replace the histamine molecule (2.396 Å) (Fig. 8a(iii)). According to the adsorption energy (1.106 eV), the exchange between histamine and C-dot is energetically favorable, suggesting that AR-COOH-Fe, AR-OH-Fe, AR-NH-Cu, AR-COOH-Cu, AR-SH-Hg/C-dots can interact favorably with histamine as a capping agent (dash-dotted line, see Supp. Mat. Fig. S10).
Natural transition orbitals (NTOs): NTOs were obtained using DFT with B3LYP/DGDZVP at (S = 1/2) at the ground state for the following systems: [AR-COOH-Fe3+-histamine], [AR-OH-Fe3+-histamine], [AR-COOH-Cu2+-histamine], [AR-NH2-Cu2+-histamine] and [AR-SH-Hg2+-histamine] and determined appropriately the delocalization properties, especially for [AR-Fe3+], [AR-COOH], [AR-COOH-Fe3+], and [AR-COOH-Fe3+-histamine] [118]. The positive hole NTOs are localized on the H atoms and metal ions, and the electron NTOs are delocalized over the σ* orbital of the functional groups (COOH, NH2, OH, and SH, and N-histamine) or π* orbital of the AR moiety (Fig. 8a and see Supp. Mat. Figs. S10, S11, S12, S13 and S14). The TD-DFT absorption spectra obtained for the above systems show their electronic transitions [107, 108] (see Fig. 8). Furthermore, the transition of electron density between the ground and excited states reveals that the electron is removed from the occupied NTO (ground state) into the excited state (virtual NTO, particle density). After analyzing carefully several electronic states (~40) for all the systems, we have detected the electronic state which is able to exhibit relatively a large oscillator strength as number 17: (f = 0.0661, λ = 578.68 nm) for [AR-COOH-Fe3+-histamine], number 35 (f = 0.1901, λ = 241.58 nm) for [AR-OH-Fe3+-histamine]; number 30: (f = 0.2857, λ = 262.31 nm) for [AR-COOH-Cu2+-histamine], number 23: f = 0.1791 λ = 417.42 nm for [AR-NH2-Cu2+-histamine] and number 35 (f = 0.3958, λ = 222.55 nm) for [AR-SH-Hg2+-histamine]. So, the orbital transition is occurred through electronic state number (87, 81) HOMO to LUMO (88, 82) for α-orbital and HOMO (state number 86, 88, 80) to LUMO (87, 89, 81) for β-orbital, and it yields the absorption maximum (578.68, 417.42 nm) for [AR-COOH-Fe3+-histamine], and (871.39 nm) for [AR-NH2-Cu2+-histamine]. The orbital transition for [AR-COOH-Cu2+-histamine], [AR-OH-Fe3+-histamine], and [AR-SH-Hg2+-histamine] was seen at electronic states such as 80, 89, 58 (HOMO) to 81, 90, 59 (LUMO), giving absorption maximum of 241.58, 262.31, 222.55 nm, respectively. These transitions are assigned to an admixture of intraligand charge transfer (ILCT) and MLCT states (charge transfer).
a NTOs, orbital 87, 80, 89, 81, 58 (particle), and orbital 88, 81, 90, 82, 59 (hole): (i) [AR-COOH-Fe3+-histamine], (ii) [AR-OH-Fe3+-histamine], (iii) [AR-COOH-Cu2+-histamine], (iv) [AR-NH2-Cu2+-histamine] and (v) [AR-SH-Hg2+-histamine], excited state number 17, 35, 30, 23, 35 at S = 1/2. b Electron density isosurface: (i) [AR-COOH-Fe3+-histamine], (ii) [AR-OH-Fe3+-histamine], (iii) [AR-COOH-Cu2+-histamine], (iv) [AR-NH2-Cu2+-histamine] and (v) [AR-SH-Hg2+-histamine] at gaseous state: the code of color for the structure C (grey), H (white), N (blue), O (red) and Fe (gray/lavender), Cu (red light) and Hg (gray light), and (contour 0.05 e Å−3), HOMO and LUMO: HOMO and LUMO contour plots (isosurface value = 0.05 au)
The electron density movement was studied by an atom-connectivity method through a molecular graph (electronic contour density), which indicates the existence of the interaction between AR with metal ions. Because the electron density of − 1.014 for N1, -0.258 for C1, and a positive electron density of 0.442 for hydrogen, -0.531for O1 in [AR-COOH] was observed in the excited states for the system [AR-COOH-Fe3+] (Fig. 8bi). The interaction of AR-OH with Fe3+ at a perpendicular position was seen for [AR-OH-Fe3+] (see Fig. 8bii). A similar type of interaction was also observed for other systems such as [AR-COOH-Cu2+], [AR-NH2-Cu2+], [AR-COOH-Cu2+-histamine], [AR-NH2-Cu2+-histamine] and [AR-SH-Hg2+-histamine] in the excited state (Fig. 8biii, iv, and v). This is consistent with the electronic contour density analysis where the existence of interaction in [AR-COOH-Fe3+], [AR-OH-Fe3+], [AR-COOH-Cu2+], [AR-NH2-Cu2+], and [AR-SH-Hg2+] as well with the histamine was detected (Fig. 8ai-v). The same observation was seen for other remaining systems such as [AR-COOH-Fe3+-histamine], [AR-OH-Fe3+-histamine], [AR-COOH-Cu2+-histamine], [AR-NH2-Cu2+-histamine] and [AR-SH-Hg2+-histamine], (Hg2+) in the excited state.
Conclusion
The transformation of plastic waste into fluorescent carbon dots (CDs) archived is characterized by different analytical methods, and then employed successfully for the recognition of Fe(III), Cu(II), or Hg(II). The results show that there is a distinct fluorescence emission caused by the interaction of the cations with CDs. It means that the fluorescence emission was turned off for the interaction of CDs with the above cations. The limit of detection was found to be as low as 0.35 µM for Cu(II), 1.38 µM for Hg(II), and 0.51 µM Fe(III). The CDs/M2+ system was then employed for sensing histamine exhibiting a “turned-on switch” and it enhances the fluorescence. It is consistent with the cell image development studies. The function CDs were analyzed by DFT using the naphthalene layer as a model for CDs and studied the interaction of metal ion detecting histamine and the obtained TD DFT spectra coincided with that of experimental spectra for CDs/M2+/histamine systems. It shows that plastic waste-based CDs can be used to detect toxic metals and histamine molecules.
Code Availability
Not applicable.
Data Availability
Not applicable.
References
Parra J, Ania C, Arenillas A, Rubiera F, Palacios J, Pis J (2004) Materiales carbonosos obtenidos a partir del reciclado de PET. Boletín-Sociedad Española de cerámica y vidrio 43:547–549
El Essawy NA, Ali SM, Farag HA, Konsowa AH, Elnouby M, Hamad HA (2017) Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol Environ Safty 145:57–68
North EJ, Halden RU (2013) Plastics and environmental health: the road ahead. Environ Health Rev 28:1–8
Dutt K, Soni R (2013) A review on synthesis of value added products from polyethylene terephthalate (PET) waste. Polym Sci Ser B 55:430–452
Mallakpour S, Behranvand V (2016) Manufacture and characterization of nanocomposite materials obtained from incorporation of d-glucose functionalized MWCNTs into the recycled poly (ethylene terephthalate). Des Monomers Polym 19:283–289
Welle F (2011) Twenty years of PET bottle to bottle recycling—An overview. Resour Conserv and Recycl 55:865–875
Xanthos D, Walker TR (2017) International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar Pollut Bull 118:17–26
Miandad R, Barakat M, Aburiazaiza AS, Rehan M, Ismail I, Nizami A (2017) Effect of plastic waste types on pyrolysis liquid oil. Int Biodeterior Biodegr 119:239–252
Rodríguez A (2017) Biodegradabilidad de materiales bioplásticos ciencia y Tecnología. de Aliment 22:11
Sharuddin SDA, Abnisa F, Daud WMAW, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manag 115:308–326
Zhuo C, Levendis YA (2014) Upcycling waste plastics into carbon nanomaterials: a review. J Appl Polym Sci 131:4
Nkwachukwu OI, Chima CH, Ikenna AO, Albert L (2013) Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries International. J Ind Chem 4:1–13
Coelho TM, Castro R, Gobbo J Jr (2011) PET containers in Brazil: Opportunities and challenges of a logistics model for post-consumer waste recycling. Resour Conserv Recycl 55:291–299
Mishra S, Goje A, Zope V (2003) Chemical recycling, kinetics, and thermodynamics of poly (ethylene terephthalate)(PET) waste powder by nitric acid hydrolysis. Polym React Eng 11:79–99
Saha B, Ghoshal A (2005) Thermal degradation kinetics of poly (ethylene terephthalate) from waste soft drinks bottles. J Chem Eng 111:39–43
Ramanan V, Siddaiah B, Raji K, Ramamurthy P (2018) Green synthesis of multifunctionalized, nitrogen-doped, highly fluorescent carbon dots from waste expanded polystyrene and its application in the fluorimetric detection of Au3 + ions in aqueous media. ACS Sustain Chem Eng 6:1627–1638
Yang Y, Yang J, Wu W-M, Zhao J, Song Y, Gao L, Yang R, Jiang L (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 1. Chemical and physical characterization and isotopic tests. Environ Sci Technol 49:12080–12086
Jan MR, Shah J, Rahim A (2008) Recovery of styrene monomer from waste polystyrene using catalytic degradation. Am Lab 40:12
Mahmoud ME, Abdou AE, Ahmed SB (2016) Conversion of waste styrofoam into engineered adsorbents for efficient removal of cadmium, lead and mercury from water. ACS Sustain Chem Eng 4:819–827
Berkmans AJ, Jagannatham M, Priyanka S, Haridoss P (2014) Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from PET wastes using the arc discharge method. J Waste Manag 34:2139–2145
Zhou Y, Sharma SK, Peng Z, Leblanc RM (2017) Polymers in carbon dots: a review. Polym 9:67
Tao S, Song Y, Zhu S, Shao J, Yang B (2017) A new type of polymer carbon dots with high quantum yield: from synthesis to investigation on fluorescence mechanism. Polym React Eng 116:472–478
Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8:355–381
Li H, Guo S, Li C, Huang H, Liu Y, Kang Z (2015) Tuning laccase catalytic activity with phosphate functionalized carbon dots by visible light. ACS Appl Mater Interface 7:10004–10012
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737
Hong G, Diao S, Antaris AL, Dai H (2015) Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev 115:10816–10906
Aji MP, Wiguna PA, Rosita N, Aisyah S (2016) Carbon nanodots from frying oil as catalyst for photocatalytic degradation of methylene blue assisted solar light irradiation. J Am Appl Sci 13:432–438
Maiaugree W, Lowpa S, Towannang M, Rutphonsan P, Tangtrakarn A, Pimanpang S, Maiaugree P, Ratchapolthavisin N, Sang-Aroon W, Jarernboon W (2015) A dye sensitized solar cell using natural counter electrode and natural dye derived from mangosteen peel waste. Sci Rep 5:1–12
Wang J, Gao M, Ho GW (2014) Bidentate-complex-derived TiO2/carbon dot photocatalysts: in situ synthesis, versatile heterostructures, and enhanced H2 evolution. J Mater Chem A
Zhu J, Bai X, Zhai Y, Chen X, Zhu Y, Pan G, Zhang H, Dong B, Song H (2017) Carbon dots with efficient solid-state photoluminescence towards white light-emitting diodes. J Mater Chem C 5:11416–11420
Lauria A, Lizundia E (2020) Luminescent carbon dots obtained from polymeric waste. J Clean Prod 262:121288
Song Y, Zhu S, Zhang S, Fu Y, Wang L, Zhao X, Yang B (2015) Investigation from chemical structure to photoluminescent mechanism: a type of carbon dots from the pyrolysis of citric acid and an amine. J Mater Chem 3:5976–5984
Bhattacharya A, Das S, Mukherjee TK (2016) Insights into the thermodynamics of polymer nanodot–human serum albumin association: a Spectroscopic and calorimetric approach. Langmuir 32:12067–12077
Yarur F, Macairan J-R, Naccache R (2019) Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ Sci Nano 6:1121–1130
Varadan VK, Chen L, Xie J (2008) Nanomedicine: design and applications of magnetic nanomaterials, nanosensors and nanosystems. John Wiley & Sons
Borah SB, Bora T, Baruah S, Dutta J (2015) Heavy metal ion sensing in water using surface plasmon resonance of metallic nanostructures. Groundw Sustain Dev 1:1–11
Sun S, Chen Q, Tang ZD, Liu C, Li ZJ, Wu AG, Lin HW (2020) Tumor microenvironment stimuli-responsive fluorescence imaging and synergistic Cancer therapy by Carbon-Dot-Cu(2+)nanoassemblies. Angew Chem Int Ed 59:21041–21048
Yu LY, Ren GJ, Tang MY, Zhu BY, Chai F, Li G, Xu DD (2018) Effective determination of Zn2+, Mn2+, and Cu2 + simultaneously by using dual-emissive carbon dots as colorimetric fluorescent probe. Eur J Inorg Chem 3418–3426
Wang Y, Kim SH, Feng L (2015) Highly luminescent N, S- co-doped carbon dots and their direct use as mercury(II) sensor. Anal Chim Acta 890:134–142
Wu LH, Long RQ, Li T, Tang C, Tong X, Guo Y, Shi SY, Xiang HY, Tong CY (2020) One-pot fabrication of dual-emission and single-emission biomass carbon dots for Cu2 + and tetracycline sensing and multicolor cellular imaging. Anal Bioanal Chem 412:7481–7489
Liu LL, Gong HR, Li DF, Zhao LN (2018) Synthesis of Carbon Dots from Pear Juice for fluorescence detection of Cu2 + ion in Water. J Nanosci Nanotechnol 18:5327–5332
Li ZL, Zhang Y, Niu QQ, Mou MY, Wu Y, Liu XX, Yan ZY, Liao SH (2017) A fluorescence probe based on the nitrogen-doped carbon dots prepared from orange juice for detecting Hg2 + in water. J Lumin 187:274–280
Yu J, Song N, Zhang YK, Zhong SX, Wang AJ, Chen JR (2015) Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2 + and Fe3+. Sens Actuators B Chem 214:29–35
Gu D, Shang SM, Yu Q, Shen J (2016) Green synthesis of nitrogen-doped carbon dots from lotus root for hg(II) ions detection and cell imaging. Appl Surf Sci 390:38–42
Wang YH, Zhang C, Chen XC, Yang B, Yang L, Jiang CL, Zhang ZP (2016) Ratiometric fluorescent paper sensor utilizing hybrid carbon dots-quantum dots for the visual determination of copper ions. Nanoscale 8:5977–5984
Ning G, Li B, Liu JJ, Xiao Q, Huang S (2022) Red-emission carbon dots as fluorescent “on-off-on” probe for highly sensitive and selective detection of Cu2 + and glutathione. Anal Bioanal Chem 414:2219–2233
Zhao L, Li HY, Xu Y, Liu HC, Zhou TY, Huang N, Li Y, Ding L (2018) Selective detection of copper ion in complex real samples based on nitrogen-doped carbon quantum dots. Anal Bioanal Chem 410:4301–4309
Liu XR, Zhang SX, Xu H, Wang RR, Dong LN, Gao SM, Tang BY, Fang WN, Hou FJ, Zhong LL, Aldalbahi A (2020) Nitrogen-Doped Carbon Quantum Dots from Poly(ethyleneimine) for Optical Dual-Mode determination of Cu2 + and L-Cysteine and their Logic Gate Operation. ACS Appl Mater Interface 12:47245–47255
Ma X, Lin SJ, Dang YF, Dai Y, Zhang XJ, Xia F (2019) Carbon dots as an “on-off-on” fluorescent probe for detection of Cu(II) ion, ascorbic acid, and acid phosphatase. Anal Bioanal Chem 411:6645–6653
Wang L, Bi YD, Gao J, Li YJ, Ding H, Ding L (2016) Carbon dots based turn-on fluorescent probes for the sensitive determination of glyphosate in environmental water samples. RSC Adv 6:85820–85828
Li HX, Su DD, Gao H, Yan X, Kong DS, Jin R, Liu XM, Wang CG, Lu GY (2020) Design of red emissive carbon dots: robust performance for analytical applications in pesticide monitoring. Anal Chem 92:3198–3205
Dong YQ, Wang RX, Li GL, Chen CQ, Chi YW, Chen GN (2012) Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal Chem 84:6220–6224
Wang WP, Lu YC, Huang H, Wang AJ, Chen JR, Feng JJ (2014) Solvent-free synthesis of sulfur-and nitrogen-co-doped fluorescent carbon nanoparticles from glutathione for highly selective and sensitive detection of mercury(II) ions. Sens Actuators B Chem 202:741–747
Ye QH, Yan FY, Luo YM, Wang YY, Zhou XG, Chen L (2017) Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion Spectrochimica. Spectrochim Acta-A: Mol Biomol 173:854–862
Wang CJ, Wang YB, Shi HX, Yan YJ, Liu EZ, Hu XY, Fan J (2019) A strong blue fluorescent nanoprobe for highly sensitive and selective detection of mercury(II) based on sulfur doped carbon quantum dots. Mater Chem Phys 232:145–151
Liu T, Dong JX, Liu SG, Li N, Lin SM, Fan YZ, Lei JL, Luo HQ, Li NB (2017) Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2 + ions detection. J Hazard Mater 322:430–436
Guo YM, Zhang LF, Cao FP, Leng YM (2016) Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci Rep 6:35795
Yan FY, Zou Y, Wang M, Mu XL, Yang N, Chen L (2014) Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells. Sens Actuators B Chem 192:488–495
Xu Y, Fan Y, Zhang L, Wang Q, Fu HY, She YB (2019) A novel enhanced fluorescence method based on multifunctional carbon dots for specific detection of Hg2 + in complex samples. Spectrochim Acta A: Mol Biomol 220:117109
Yan FY, Shi DC, Zheng TC, Yun KY, Zhou XG, Chen L (2016) Carbon dots as nanosensor for sensitive and selective detection of Hg2 + and L-cysteine by means of fluorescence “Off-On” switching. Sens Actuators B Chem 224:926–935
Dickinson AW, Power A, Hansen M, Brandt K, Piliposian G, Appleby P, O’neill P, Jones R, Sierocinski P, Koskella B (2019) Heavy metal pollution and co-selection for antibiotic resistance: a microbial palaeontology approach. Environ Int 132:105117
Cittan M, Tirtom VN, Dincer A, Celik A (2014) Epichlorohydrin crosslinked chitosan-clay composite beads for on-line preconcentration and determination of chromium(III) by flow injection flame atomic absorption spectrometry. Anal Method 6:5298–5303
Kamakura N, Inui T, Kitano M, Nakamura T (2014) Determination of chromium(III), chromium(VI), and chromium(III) acetylacetonate in water by ion-exchange disk extraction/metal furnace atomic absorption spectrometry. Spectrochim Acta B 93:28–33
Reddy MA, Meeravali NN, Kumar SJ (2009) Comparison of the Cloud Point extraction and Matrix volatilization procedures for the determination of Cr, Cu, Fe, Ni, and Pb in a Germanium Matrix by Graphite furnace atomic absorption spectrometry. Atom Spectr 30:92–97
Diao QP, Ma PY, Lv LL, Li TC, Wang XH, Song DQ (2016) A novel fluorescent probe for Cr3 + based on rhodamine-crown ether conjugate and its application to drinking water examination and bioimaging. Spectrochim Acta A: Mol Biomol 156:15–21
Meeravali NN, Jiang SJ (2009) A novel cloud point extraction approach using cationic surfactant for the separation and pre-concentration of chromium species in natural water prior to ICP-DRC-MS determination. Talanta 80:173–178
Zhang N, Suleiman JS, He M, Hu B (2008) Chromium(III)-imprinted silica gel for speciation analysis of chromium in environmental water samples with ICP-MS. Detect Talanta 75:536–543
Kachoosangi RT, Compton RG (2013) Voltammetric determination of chromium(VI) using a gold film modified carbon composite electrode. Sens Actuators B Chem 178:555–562
Wang R, Wang X, Sun Y (2017) One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens Actuators B Chem 241:73–79
Hu C, Liu Y, Yang Y, Cui J, Huang Z, Wang Y, Yang L, Wang H, Xiao Y, Rong J (2013) One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J Mater Chem B1:39–42
Yang Z, Xu MH, Liu Y, He FJ, Gao F, Su YJ, Wei H, Zhang YF (2014) Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 6:1890–1895
Sun GL, Li XJ, Qu YD, Wang XH, Yan HH, Zhang YJ (2008) Preparation and characterization of graphite nanosheets from detonation technique. Mater Lett 62:703–706
Sun G, Li X, Qu Y, Wang X, Yan H, Zhang Y (2008) Preparation and characterization of graphite nanosheets from detonation. technique Mater Lett 62:703–706
Chaudhary S, Kumari M, Chauhan P, Chaudhary GR (2021) Upcycling of plastic waste into fluorescent carbon dots: an environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag 120:675–686
Veerakumar P, Salamalai K, Thanasekaran P, Lin K-C (2018) Simple preparation of porous carbon-supported ruthenium: propitious catalytic activity in the reduction of ferrocyanate (III) and a cationic dye. ACS Omega 3:12609–12621
Chaudhary S, Kumar S, Kaur B, Mehta S (2016) Potential prospects for carbon dots as a fluorescence sensing probe for metal ions. RSC Adv 6:90526–90536
Niu J, Gao H, Wang L, Xin S, Zhang G, Wang Q, Guo L, Liu W, Gao X, Wang Y (2014) Facile synthesis and optical properties of nitrogen-doped carbon dots. New J Chem 38:1522–1527
Lu S, Sui L, Liu J, Zhu S, Chen A, Jin M, Yang B (2017) Near-infrared photoluminescent polymer–carbon nanodots with two‐photon fluorescence. Adv Mater 29:1603443
Kharangarh PR, Umapathy S, Singh G (2018) Investigation of sulfur related defects in graphene quantum dots for tuning photoluminescence and high quantum yield. Appl Surf Sci 449:363–370
Kumari M, Chaudhary GR, Chaudhary S, Umar A (2021) Rapid analysis of trace sulphite ion using fluorescent carbon dots produced from single use plastic cups. Eng sci 17:101–112
Hu Y, Gao Z, Yang J, Chen H, Han L (2019) Environmentally benign conversion of waste polyethylene terephthalate to fluorescent carbon dots for “on-off-on” sensing of ferric and pyrophosphate ions. J Colloid Interface Sci 538:481–488
Hu Y, Yang J, Tian J, Jia L, Yu J-S (2014) Green and size-controllable synthesis of photoluminescent carbon nanoparticles from waste plastic bags. RSC Adv 4:47169–47176
Cruz MISD, Thongsai N, de Luna MDG, In I, Paoprasert P (2019) Preparation of highly photoluminescent carbon dots from polyurethane: optimization using response surface methodology and selective detection of silver (I) ion. Colloids Surf A Physicochem Eng Asp 568:184–194
Hong D, Deng X, Liang J, Li J, Tao Y, Tan K (2019) One-step hydrothermal synthesis of down/up-conversion luminescence F-doped carbon quantum dots for label-free detection of Fe3+. Microchem J 151:104217
Khan ZM, Rahman RS, Islam S, Zulfequar M (2019) Hydrothermal treatment of red lentils for the synthesis of fluorescent carbon quantum dots and its application for sensing Fe3+. Opt Mater 91:386–395
Zhang Z, Sun W, Wu P (2015) Highly photoluminescent carbon dots derived from egg white: facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain Chem Eng 3:1412–1418
Kailasa SK, Ha S, Baek SH, Kim S, Kwak K, Park TJ (2019) Tuning of carbon dots emission color for sensing of Fe3 + ion and bioimaging applications. Mater Sci Eng C 98:834–842
Wang N, Wang Y, Guo T, Yang T, Chen M, Wang J (2016) Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens Bioelectron 85:68–75
Ma H, Sun C, Xue G, Wu G, Zhang X, Han X, Qi X, Lv X, Sun H, Zhang J (2019) Facile synthesis of fluorescent carbon dots from Prunus cerasifera fruits for fluorescent ink, Fe3 + ion detection and cell imaging. Spectrochim Acta A Mol Biomol Spectrosc 213:281–287
Shen J, Shang S, Chen X, Wang D, Cai Y (2017) Facile synthesis of fluorescence carbon dots from sweet potato for Fe3 + sensing and cell imaging. Mater Sci Eng C 76:856–864
Zulfajri M, Gedda G, Chang C-J, Chang Y-P, Huang GG (2019) Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3 + ions in aqueous solution. ACS Omega 4:15382–15392
Qi H, Teng M, Liu M, Liu S, Li J, Yu H, Teng C, Huang Z, Liu H, Shao Q (2019) Biomass-derived nitrogen-doped carbon quantum dots: highly selective fluorescent probe for detecting Fe3 + ions and tetracyclines. J Colloid Interface Sci 539:332–341
Zhao J, Huang M, Zhang L, Zou M, Chen D, Huang Y, Zhao S (2017) Unique approach to develop carbon dot-based nanohybrid near-infrared ratiometric fluorescent sensor for the detection of mercury ions. Anal Chem 89:8044–8049
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, Asiri AM, Al-Youbi AO, Sun X (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal Chem 84:5351–5357
Xu Y, Fan Y, Zhang L, Wang Q, Fu H, She Y (2019) A novel enhanced fluorescence method based on multifunctional carbon dots for specific detection of Hg2 + in complex samples. Spectrochim Acta A: Mol Biomol Spect 220:117109
Guo Y, Zhang L, Cao F, Leng Y (2016) Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci Rep 6:1–7
Li X, Pan L, Yang F, Yang L (2022) Blue Carbon dot-based portable smartphone platform for visualization of copper (II). ACS Appl Nano Mater 5:9252–9259
Lu W, Gao Y, Jiao Y, Shuang S, Li C, Dong C (2017) Carbon nano-dots as a fluorescent and colorimetric dual-readout probe for the detection of arginine and cu 2 + and its logic gate operation. Nanoscale 9:11545–11552
Ma X, Dong Y, Sun H, Chen N (2017) Highly fluorescent carbon dots from peanut shells as potential probes for copper ion: the optimization and analysis of the synthetic process. Mater Today Chem 5:1–10
Kumari A, Kumar A, Sahu SK, Kumar S (2018) Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2 + ion sensing and live cell imaging. Sens Actuators B Chem 254:197–205
Bootsma AN, Doney AC, Wheeler SE (2019) Predicting the Strength of stacking interactions between Heterocycles and aromatic amino acid side chains. J Am Chem Soc 141:11027–11035
Sanchez-Flores EI, Chavez-Calvillo R, Keith TA, Cuevas G, Rocha-Rinza T, Cortes-Guzman F (2014) Properties of Atoms in electronically excited molecules within the formalism of TDDFT. J Comput Chem 35:820–828
Yang W, Mortier WJ (1986) The use of global and local molecular-parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711
Lee CT, Yang WT, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B 37:785–789
Raeyani D, Shojaei S, Ahmadi-Kandjani S (2018) Optical graphene quantum dots gas sensors. Theoretical study Superlattices and Microstructures 114:321–330
Pal T (2018) Facile and green synthesis of Multicolor fluorescence Carbon Dots from Curcumin: in Vitro and in vivo bioimaging and other applications. ACS Omega 3:831–843
Wang YF, Zhu YW, Yu SM, Jiang CL (2017) Fluorescent carbon dots: rational synthesis, tunable optical properties and analytical applications. RSC Adv 7:40973–40989
Liu HF, Li ZH, Sun YQ, Geng X, Hu YL, Meng HM, Ge J, Qu LB (2018) Synthesis of luminescent Carbon dots with Ultrahigh Quantum yield and inherent folate receptor-positive. Cancer Cell Targetability Sci Rep 8:1086
Choi Y, Choi Y, Kwon OH, Kim BS (2018) Carbon Dots: Bottom-Up Syntheses, Properties, and light-harvesting applications. Chem Asian J 13:586–598
Cui MJ, Ren SM, Zhao HC, Wang LP, Xue QJ (2018) Novel nitrogen doped carbon dots for corrosion inhibition of carbon steel in 1 M HCl solution. Appl Surf Sci 443:145–156
Xie SY, Huang RB, Zheng LS (1999) Separation and identification of perchlorinated polycyclic aromatic hydrocarbons by high-performance liquid chromatography and ultraviolet absorption spectroscopy. J Chromatogr A 864:173–177
Atkins PW, Friedman RS (2005) Molecular quantum mechanics
Sakamoto K, Nishina N, Enoki T, Aihara J (2014) Aromatic character of Nanographene Model Compounds. J Phy Chem A 118:3014–3025
Fujii S, Enoki T (2013) Nanographene and Graphene Edges: electronic structure and nanofabrication. Acc Chem Res 46:2202–2210
Sun XM, Li YD (2004) Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew Chem Int Ed 43:597–601
Li M, Li W, Liu SX (2012) Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method. J Mat Res 27:1117–1123
Sevilla M, Fuertes AB (2009) Chemical and Structural Properties of Carbonaceous Products obtained by Hydrothermal Carbonization of Saccharides. Eur J Chem 15:4195–4203
Li L, Fu ML, Yang DY, Tu YF, Yan JL (2022) Sensitive detection of glutathione through inhibiting quenching of copper nanoclusters fluorescence. Spectrochim Acta A 267:120563
Acknowledgements
The acknowledgments come at the end of an article after the conclusions and before the notes and references. The authors gratefully acknowledge the DGSCA-UNAM for the computational facilities. The authors acknowledge the Dirección General de Asuntos del Personal Académico (Project PAPIIT No IN202622) for the economic support. Jessica Muro-Hidalgo thanks the CONACYT for her master’s degree fellowship to do this work, and Luciano Hernández-Gómez for the technical support in the cell imaging confocal studies.
Funding
Dirección General de Asuntos de Personal Académico (Project PAPIIT No IN202622).
Author information
Authors and Affiliations
Contributions
Jessica M. Muro-Hidalgo: Master student, worked in the fluorescence experiments and analysis; Iván J. Bazany-Rodríguez: Revision of fluorescence studies and write up and interpretations, José Guadalupe Hernández: DFT studies and analysis; Victor Manuel Luna Pabello: Confoal studies of cell imaging, and Pandiyan Thangarasu: Manuscript writing, data analysis, and revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors gave their consent for publication.
Conflict of Interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muro-Hidalgo, J.M., Bazany-Rodríguez, I.J., Hernández, J.G. et al. Histamine Recognition by Carbon Dots from Plastic Waste and Development of Cellular Imaging: Experimental and Theoretical Studies. J Fluoresc 33, 2041–2059 (2023). https://doi.org/10.1007/s10895-023-03201-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03201-7