Abstract
The neuro-stimulant anti-narcoleptic drug as modafinil (MOD) is used to treatment neurological conditions caused by COVID-19. MOD was used to treatment narcolepsy, shift-work sleep disorder, and obstructive sleep apnea-related sleepiness. So, an innovative, quick, economical, selective, and ecologically friendly procedure was carried out. A highly sensitive N@CQDs technique was created from green Eruca sativa leaves in about 4 min using microwave synthesis at 700 w. The quantum yield of the synthesized N@CQDs was found to be 41.39%. By increasing the concentration of MOD, the quantum dots' fluorescence intensity was gradually quenched. After being excited at 445 nm, the fluorescence reading was recorded at 515 nm. The linear range was found to be in the range 50 – 700 ng mL−1 with lower limit of quantitation (LOQ) equal to 45.00 ng mL−1. The current method was fully validated and bio analytically according to (US-FDA and ICH) guidelines. Full characterization of the N@CQDs has been conducted by high resolution transmission electron microscope (HRTEM), Zeta potential measurement, fluorescence, UV–VIS, and FTIR spectroscopy. Various experimental variables including pH, QDs concentration and the reaction time were optimized. The proposed study is simply implemented for the therapeutic drug monitoring system (TDMS) and various clinical laboratories for further pharmacokinetic research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A neurological condition known as narcolepsy is characterized by an inability to control sleep–wake cycles. Narcoleptics may fall asleep at inappropriate times and experience daytime fatigue [1].
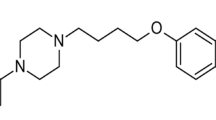
Modafinil (MOD, Fig. 1a) is (2-[(diphenylmethyl)sulfinyl] acetamide), MOD is being approved to treat narcolepsy, obstructive sleep apnea, and shift work sleep disorder, MOD is a special CNS stimulant, it differs from other central nervous system stimulants therapeutically and pharmaceutically in that it generates long-lasting waking effects without behavioral alteration, addictive qualities, or sleep rebound [1].
Various analytical methods were reported for estimation of MOD as HPLC [1,2,3,4,5,6], UPLC [7], HPTLC [8], fluorimetric [9], spectrophotometry [10, 11] and capillary electrophoresis [12].
In the present study, novel, simple, fast synthesis, environmentally friendly, organic solvents free, ultra-sensitive spectrofluorimetric approach using nitrogen from natural source to be doped in quantum dots. The proposed method (N@CQDs) provides ultra-sensitive (50 – 700 ng mL−1) and it was selective for estimation of MOD in pharmaceutical dosage form, content uniformity and human plasma than other reported fluorimetric method (0.5 – 20 µg mL−1) [9]. The N@CQDs easily and rapid synthesized from green source using microwave synthesis in 4 min with high quantum yield 41.39%.
Carbon quantum dots (CQDs), which have distinct optical properties, great water solubility, biocompatibility, non-toxicity, and simplicity of functionalization, have become well-established as an effective analytical sensor in the last ten years. Due to their remarkable and customizable fluorescence properties, CQDs are presently positioned as an excellent replacement for fluorescent dyes and fluorescence derivatizing agents. They are also less harmful to the environment and less poisonous than nanomaterials made of metal [13,14,15].
Furthermore, different techniques were utilized for synthesis of CQDs as dry heat, hydrothermal and solvothermal methods. However, these methods have various drawbacks as go against the idea of "green chemistry," as they can take up to 24 h, need temperatures up to 300 degrees, and use harsh chemicals and organic solvents. [16] Microwave synthesis quantum dots is a new approach recently applied to reduce the synthesis time form hours to minutes and greenness synthesis with quantum yield product. [17, 18] Eruca Sativa leaves is very cheap plant widely growing in Egypt. It is the oldest leaf vegetables consumed by humans. Eruca Sativa leaves are rich with various components and vitamins as carbohydrates, sugar, fibers, vitamin A, vitamin B1, riboflavin and folic acid.
The proposed approach aims to create an integrate strategy for greening both the synthesis process and the carbon source in this work in order to recover the greenness and sustainability of the synthesis of CQDs. Only low-power microwave-assisted synthesis at 700 W for 4 min is used in the suggested technique. We also had access to inexpensive, abundant, and practical plants. Additionally, this study's greenness is consistent with global claims about green chemistry and safety.
Experimental
Materials and Reagents
Modafinil (MOD 99.98%) authentic powder was obtained from Mash Premiere, Region 6, Second District, 90 Street, Fifth Settlement, New Cairo City, Egypt. Bravamax® (200 mg tablets) was purchased from the local market, Egypt. Human plasma samples were obtained from Egyptian Blood Bank and stored at -24 °C until analysis.
Standard solution of MOD (100 µg mL−1) was prepared using via 10 mg of MOD was dissolving 100 mL methanol.
Equipment’s of the N@CQDs Method
The results were acquired by an FS5 spectrofluorometer (Edinburgh, UK) with a 150 W xenon lamp source for excitation. Also, with 1-cm quartz cell and connected to Fluoracle® software. The slit widths were set to 2 nm and the scanning speed 1000 nm/min. The dynamic light scattering measurements (DLS) were scanned by Zetasizer Red badge instrument of ZEN 3600 (Malvern, UK). MFMI-100A (MED Future) Microwave instrument (2450 MHz, 0- 1000 W) was designed for catalyzing organic synthesis and solvent extraction. Magnetic and Mechanical stirring- IR Temperature Sensor (0–300'C). Fourier-transform infrared (FTIR) Germany. pH-meter (China). The powder X-ray diffraction (PXRD) was scanned by Philips X-ray diffractometer. High-resolution transmission electron microscope (HR-TEM) images were captured via JEOL JEM-100CX II unit tungsten EM filament 120 (USA).
Synthesis of Environmentally Green Quantum Dots (N@CQDs)
The green nitrogen carbon quantum dots (N@CQDs) were synthesized using thermolysis of Eruca Sativa leaves. The leaves were crushed well and filtrate, then 40 mL of the filtrate was transferred into reaction vessel and then placed in microwave. Microwave source: 2450 MHz, 0- 1000 W for 4 min until brown solution was formed. The residue was dispersed and then sonicated for 30 min to remove large particles. The solution was filtered and centrifuged at 4000 rpm for 10 min. the supernatant was filtrated via 0.45 μm cellulose membrane. The obtained yellow filtrate color solution was utilized for experiment.
Fluorimetric Analysis of MOD
One milliliter of N@CQDs (0.15 mg mL−1) was mixed with 1.0 mL of Britton-Robinson (BR) buffer (pH 7) into 5-mL volumetric flask, then 1 mL of working solution of MOD was added to obtain the final concentration range (50 – 700 ng mL−1). The resulted mixture completed by ultra-pure distilled water till the marked volume. The fluorescence intensity was measured at λem 515 nm after 10 min (excitation 445 nm).
Estimation in Pharmaceutical Product and Content Uniformity Test
Ten Bravamax® tablets (200.0 mg/tablet) were weighed, crushed finally and thoroughly mixed. Then, an amount equivalent to 10 mg MOD was transferred into a volumetric flask and then dissolved into 50 mL of methanol. The solution was sonicated about 20 min followed by filtration, then volume was completed to 100 mL with methanol to get concentration of 100 µg mL−1.
For content uniformity test [19,20,21], each tablet of Bravamax® drug was individually weighed and finally powdered. An amount equivalent to 10 mg from the powder were dissolved into 50 mL methanol with sonication for 20 min followed by filtration to remove undissolved excipients then volume completed to 100 mL methanol to get concentration of 100 µg mL−1. Then the analytical procedure was followed.
Preparation of Spiked Plasma
Into a centrifuge tube, 1.0 mL of human plasma was spiked with adequate amount of MOD solution. then furtherly 1 mL of methanol were added as protein precipitating agent [22, 23]. The mixture was vortexed for 30 s and then completed to 10 mL. The mixture was centrifuged for 30 min (3500 rpm), after that 1.0 mL of supernatant was used in analytical procedure.
Preparation of Real Sample
Real human plasma samples were conducted in accordance with the responsible committee's ethical guidelines and the 2008 revision of the 1975 Helsinki Declaration. BRAVAMAX® tablets (200.0 mg/tablet) were administrated as single oral dose by 5 healthy volunteers. The blood samples were collected after time intervals (0.25, 0.5, ……to 20 h) into heparinized tubes. The blood samples were centrifuged at 5000 rpm for 30 min to separate the plasma. 1.0 mL of the plasma was mixed with 1 ml of methanol as protein precipitating agent. Then the centrifugation was carried out at 3500 rpm for 30 min to separate the supernatant. The supernatant was utilized in analytical procedure.
Results and Discussion
Morphological Characteristics of the Quantum Dots
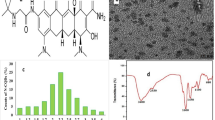
The surface morphology of N@CQDs was studied using high-resolution transmission electron microscope (HR-TEM). The size of N@CQDs was found to be 2.0 nm ± 0.21. Figure 1b
Dynamic light scattering (DLS) was carried out for particle size confirmation. The size was found to be 2.5 nm, which agrees with HR-TEM image. Fig. S1 (Supplementary Materials). Besides, the powder X-ray diffraction (PXRD) image was utilized to study the formation of N@CQDs, the peak presented at 24.60o is a diagnostic peak of carbon dots [13, 15] as seen in Fig. S2.
As shown in Fig. S3, the energy dispersive X-ray spectrometer (EDX) was carried out to check the presence of (C, N and O) elements. The spectrum shows the presence C, N, and O elements. The function groups formation on the surface of N@CQDs were examined using FTIR spectroscopy Fig. S4. The FTIR peaks appear at 3410 cm−1 and 2900 cm−1 corresponding to (-NH, -OH) and 2900 cm−1–CH groups respectively. The peaks at 1690 and 1560 cm−1 correspond to –C = O and –C = C groups. In addition, the peaks at 1556 and 1293 cm−1 refer to N–O and C–O stretching Fig. S4.
Furthermore, X-Ray photoelectron spectroscopy (XPS) was carried out for the elemental analysis. The XPS peaks of N@CQDs were observed as three characteristic strong peaks at 284.9, 395.6, and 538.5 eV corresponding to C 1 s, N 1 s, and O 1 s, respectively. It signifies that C-dots are formed from O (43.00%), C (37.89%), and N (19.11%). Fig. S5a.
The results refer to formation of N (19.11%) with percent higher than previously reported methods (6.88%, 9.15%) [24] due to surface passivation of carbon dots particles. In C 1 s spectrum (Fig. S5b) there are four peaks were observed at 284.5, 285.2, 286.4, and 288.5 eV, due to presence C = C, C-N, C-O, and C = O groups, respectively. For N 1 s spectrum, 2 peaks are observed at 399.2 eV and 400.8 eV, produced due to presence of C-N and N–H as shown in Fig. S5c [25].
For O 1 s spectrum has two peaks for C–OH, C–O–C and C = O at 531.4 eV and 532.6 eV (Fig. S5d) [13, 26]. The morphological characters of quantum dots demonstrate the structure of N@CQDs contain numerous of function groups, which interpretate the interaction between MOD and N@CQDs via electrostatic interaction and hydrogen bonding.
The quantum yield (QY) of N@QDs was studied via single point method using the following equation:
Q is the quantum yield while F is integrated fluorescence.
The proposed method provides high quantum yield due to reducing the sizes of N@CQDs (2.0 nm) would increase the quantum yields by creating more optical effects via increasing number of function groups on the surface of quantum dots. [27, 28] Furthermore, Eruca Sativa leaves are rich with various components and vitamins as carbohydrates, sugar, fibers, vitamin A, vitamin B1, riboflavin and folic acid which led to numerous and varying function groups during quantum dots synthesis. The quantum yield of amine quantum dots was found to be = 41.39%.
Optical Characters of the Green Synthesized Quantum Dots
The green N@CQDs showed two UV spectra at 229 and 296 nm as in Fig. 2. These peaks were referred to π-π* electronic transition of C = C and n-π* electronic transition of C = O related to amine carbon dots surface. Moreover, N@CQDs produce emission peak at 515 nm (λex 445 nm), which indicate optical characters of carbon quantum dots Fig. 2.
The relative fluorescence spectra of N@CQ-dots were scanned with increased excitation wavelengths from 410 to 490 nm, the increasing excitation wavelengths led to a red shift in the emission spectra followed by a decrease in RFI, confirming carbon dots excitation-dependent emission Fig. 3a.
Moreover, the effect of temperature in the range of (25 – 60 o C) on relative fluorescence intensity (RFI) of N@CQDs was studied. Increasing temperature beyond 25 o C declined the fluorescence intensity [13, 15].
Optimization of the Methodology
The pH effect on N@CQD RFI in the presence and absence of MOD was investigated, due to the presence of various function groups, N@CQDs were observed to quench steadily in the pH range of 6.8 to 7.4 and raising the pH to 7.5 caused an unstable decrease in RFI. So, the ideal pH was determined to be 7 Fig. 3b.
Furthermore, Various concentrations of N@CQDs were tested during the reaction with MOD (300 ng mL−1), it was found that 0.15 mg mL−1 (1.0 mL) produce most stable quenching Fig. 3c.
The time of the reaction of MOD with N@CQDs in pure form was studied at different time intervals ranging from 0 to 20 min. The maximum stable quenched was observed within 10 min Fig. 3d.
Reaction Validation of MOD with N@CQDs Validation
The presented approach for the reaction of modafinil in the presence of N@CQDs was validated at optimum conditions using International Conference of Harmonization (ICH) and US-FDA rules [29, 30]. The green synthesized N@CQDs fluoresence was quenched with inceasing modafinil concentration at 515 nm (excitation at 445 nm) Fig. 4a.
The regression equation was found to be Y = 0.0026x + 0.8307 using the Stern–Volmer equation, good linearity was established within the linear range of 50–700 ng mL−1, with a correlation coefficient is 0.9995 Fig. 4b.
where, F0 and F are the fluorescence efficiencies of the N@CQDs in the absence and presence of the MOD, respectively. [Q] is MOD concentration and Ksv is the Stern–Volmer constant.
The presented is ultra-sensitive one with limit of detection (LOD) value 14.85 ng mL−1 and limit of quantitation (LOQ) 45.0 ng mL−1 as seen in Table 1.

 is standard deviation.
is standard deviation.
Different concentrations form MOD within the calibration range (50, 100.0, 300.0, 500.0, and 700.0 ng mL−1) were employed to examine the N@CQDs method's accuracy. According to Table 2, the RSD values ranged from 0.40 to 1.12, and the recovery percentage ranged from 99.68 to 102.11%. The reproducibility of the presented method was further examined using three replicates of each concentration (100.0, 300.0, and 500.0 ng mL−1). According to Table 2, the RSD value was (0.16 to 1.11), showing high reproducibility of N@CQDs method.
A bio-analytical validation of the reaction of MOD with N@CQDs in human plasma was examined via three concentrations (100, 300, and 600 ng mL-1) according to US-FDA recommendation. The RSD value ranged from 1.79 to 2.33, as reported in Table 3, the results indicate to high accuracy of N@CQDs method in human plasma.
Furthermore, the robustness of the green synthesized N@CQDS was employed by making slight adjustments to the analytical procedure's parameters. As can be seen in Table 4, there was no significant effect for a slight modification in the technique variables.
In addition to the stability of modafinil in human plasma was examined using N@CQDs, as shown in Table 5. The stability was studied using three levels: low-quality control (LQC), medium-quality control (MQC) and high-quality samples (HQC) under different conditions. The outcomes refer to that modafinil is stable in human plasma under different condition. Table 5.
To further assess the accuracy and precision of the incurred plasma sample of modafinil incurred sample reanalysis (ISR) was performed. The range of values between the original samples and the incurred samples was found between 3.35 and 5.84%, according to Table 6.
The selectivity of N@CQDs was performed to evaluate the excipient interference. Talc, starch, mannitol, magnesium stearate, lactose, sodium chloride, and other excipients were evaluated alongside MOD. The findings demonstrated that the excipients had no effect, demonstrating the excellent selectivity of the indicated approaches Table 7.
Suggested Reaction Mechanism of the Proposed Method
The reaction mechanism between modafinil and N@CQDs was interpretated with Stern–Volmer equation as:
F0/F = 1 + Ksv [Q], The quenching mechanism is dynamic clearly indicated by the linearity of the Stern–Volmer figure. modafinil interacts with excited N@QDs, causing energy/electron transfer and dimming of the quantum dots' fluorescence. The Stern–Volmer model perfectly describes this process Fig. 4b.
In addition to allowing for the creation of hydrogen bonds and electrostatic attraction between MOD and nitrogen dopped carbon dots, the existence of numerous function groups in MOD allow for these other phenomena [13, 31].
Applications of N@CQDs in Human Plasma
The N@CQDs method's ultra-sensitivity enables the detection of MOD in human plasma that has been spiked. The investigated procedure at six distinct concentration levels applied was found to have a recovery percentage that ranged from 95.25% to 98.34%. The SD of the data was in the range of 0.97 to 2.17, which is within the permissible bounds of the analytical method variability resulting from various matrix effects as indicated in Table 8.
Modafinil is neuro-stimulants antinarcoleptic drug that help with the post-COVID neurological syndrome. The pharmacokinetic of MOD was investigated via healthy human volunteers, the maximum plasma concentration was found to be Cmax was determined to be 4.15 ± 0.67 µg mL−1, and tmax is 2.0 ± 0.55 h, t1/2 equal to 12.30 h and area under curve was found to be (AUC0-ꝏ) 60.33 ± 10.4 µg.h mL−1. The results were displayed in Table 9 and were closely related to the approach that had previously been reported [32].
Applications in Pharmaceutical Dosage form and Content Uniformity Test
The recommended method (N@CQDs) was used with effectiveness for quantifying MOD in commercial tablets (BRAVAMAX® 200 mg). The percentage of recovery ± SD was discovered to be 102.33 ± 1.05 as compared with reported method [9] (99.44 ± 1.09). Additionally, the results of the t-test and F-test were found to be 1.61 and 2.91, respectively. At 95% confidence, the results did not significantly differ between the proposed and reported method.
Each unit in a batch should have a drug substance composition that falls within a specific range around the label claim in to guarantee the uniformity of dosing units. For MOD, the method was ideally suited for content uniformity testing, which is a time-consuming process when using conventional assay techniques. This was because the proposed method has a high sensitivity and can quickly and accurately measure the fluorescence intensity of a single tablet extract. The test's phases were implemented in accordance with USP protocol [33]. When the acceptance value (AV) was calculated, it was discovered that it was lower than the permitted maximum acceptance value (L1). As indicated in Table 10, the outcomes showed good medication consistency.
Assessment of the Greenness of the Proposed Method Versus Reported Method
Several assessment tools have been recently reported for evaluation of the ecological impacts of the analytical methodologies. The assessment of analytical methods helps in reduction of environmental pollution generated by such processes. For instance, an average of 0.5L of organic waste is generated daily from a conventional HPLC system [34] therefore, the greenness assessment became a must do evaluation. Since the proposed method is suitable for application in determining MOD in pharmaceutical dosage forms as well as plasma samples, the method is to be compared to another previously reported method [2]. As seen in Table 11, two green assessments were utilized for estimation the greenness of the proposed method GAPI and AGREE methods [35,36,37]. The results in Table 11 refer to high greenness effect of the proposed method (N@CQDs) which agree with US-climate change conference.
Conclusion
The presented study provides novel, simple, environmentally friendly, highly sensitive, economical, and quick approach for the synthesis of N@CQDs as fluorescent probes from green source. A one-pot, low-energy, chemical-free carbonization utilizing a conventional microwave has been used to quickly develop the N@CQDs. The suggested approach was validated, and bio analytically validated using ICH and US-FDA requirements. In both a content uniformity test and a pharmacokinetic investigation, this sensor was successfully used to determine MOD. As a result, this straightforward and label-free sensing platform was used as the fluorescence-based assessment of the target analyte without the need for chemical derivatization or the extensive reaction times that are required by previously published methods.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Schwertner HA, Kong SB (2005) Determination of modafinil in plasma and urine by reversed phase high-performance liquid-chromatography. J Pharm Biomed Anal 37:475–479. https://doi.org/10.1016/j.jpba.2004.11.014
Gorman SH (2002) Determination of modafinil, modafinil acid and modafinil sulfone in human plasma utilizing liquid–liquid extraction and high-performance liquid chromatography. J Chromatogr B 767:269–276. https://doi.org/10.1016/S1570-0232(01)00572-4
Moachon G, Matinier D (1994) Simultaneous determination of modafinil and its acid metabolite by high-performance liquid chromatography in human plasma. J Chromatogr B Biomed Sci Appl 654:91–96. https://doi.org/10.1016/0378-4347(93)E0452-V
Burnat P, Robles F, Do B (1998) High-performance liquid chromatographic determination of modafinil and its two metabolites in human plasma using solid-phase extraction. J Chromatogr B 706:295–304. https://doi.org/10.1016/S0378-4347(97)00550-1
Bhimanadhuni CNR, Garikapati DR, Karamsetty S (1970) Development and Validation of RP-HPLC method for determination of Modafinil in bulk and dosage form. Int Curr Pharm J 1:77–80. https://doi.org/10.3329/icpj.v1i4.10062
Younus M, Arif M, Richards M, Kumar B (2013) Determination of Venlafaxine and Modafinil in Individual Tablet Dosage Forms using Single RP-HPLC Method. Trop J Pharm Res 12:239–245. https://doi.org/10.4314/tjpr.v12i2.17
He Y, Ma Y, Luo L et al (2022) Determination of modafinil in rat plasma by UPLC-MS/MS and a study of its pharmacokinetics and bioavailability. Acta Chromatogr 1–5. https://doi.org/10.1556/1326.2022.01041
Gaurang P, Pandya GPP (2013) Stability Indicating HPTLC Method for Estimation of Modafinil in the Bulk and Tablet Formulation. IOSR J Pharm Biol Sci 5:22–28. https://doi.org/10.9790/3008-0552228
Hosny NM, Gadallah MI, Gomila RM, Qayed WS (2022) Innovative computationally designed-spectrofluorimetric method for determination of modafinil in tablets and human plasma. Talanta 236:122890. https://doi.org/10.1016/j.talanta.2021.122890
Elsheikh SG, El-Mosallamy SS, Fayez YM, Hassan AME (2022) Smart stability indicating spectrophotometric methods for determination of modafinil: the promising treatment for post-covid neurological syndrome. BMC Chem 16:79. https://doi.org/10.1186/s13065-022-00869-z
Bijithra C, Ragan G, Shanmugasundaram P (2016) Analytical Method Development and Validation of Modafinil in Pure and Tablet Dosage Form by UV Spectroscopy. Res J Pharm Technol 9:1303–1308. https://doi.org/10.5958/0974-360X.2016.00202.X
al Azzam KM, Saad B, Adnan R, IdirisSaleh M (2009) Enantioselective determination of modafinil in pharmaceutical formulations by capillary electrophoresis, and computational calculation of their inclusion complexes. Microchimica Acta 166:311–317. https://doi.org/10.1007/s00604-009-0209-4
Salman BI, Ibrahim AE, El Deeb S, Saraya RE (2022) Fabrication of novel quantum dots for the estimation of COVID-19 antiviral drug using green chemistry: application to real human plasma. RSC Adv 12:16624–16631. https://doi.org/10.1039/d2ra02241a
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM (2020) One pot fabrication of fluorescein functionalized manganese dioxide for fluorescence “Turn OFF–ON” sensing of hydrogen peroxide in water and cosmetic samples. RSC Adv 10:17506–17514. https://doi.org/10.1039/D0RA01980A
Salman BI, Hassan YF, Eltoukhi WE, Saraya RE (2022) Quantification of tyramine in different types of food using novel green synthesis of ficus carica quantum dots as fluorescent probe. Luminescence 37:1259–1266. https://doi.org/10.1002/bio.4291
Wang X, Feng Y, Dong P, Huang J (2019) A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front Chem 7:671. https://doi.org/10.3389/fchem.2019.00671
Choi Y, Thongsai N, Chae A et al (2017) Microwave-assisted synthesis of luminescent and biocompatible lysine-based carbon quantum dots. J Ind Eng Chem 47:329–335. https://doi.org/10.1016/j.jiec.2016.12.002
Eskalen H, Uruş S, Cömertpay S et al (2020) Microwave-assisted ultra-fast synthesis of carbon quantum dots from linter: Fluorescence cancer imaging and human cell growth inhibition properties. Ind Crops Prod 147:112209. https://doi.org/10.1016/j.indcrop.2020.112209
Omar MA, Hammad MA, Salman BI, Derayea SM (2016) Highly sensitive spectrofluorimetric method for determination of doxazosin through derivatization with fluorescamine; Application to content uniformity testing. Spectrochim Acta A 157:55–60. https://doi.org/10.1016/j.saa.2015.12.012
Saraya RE, Hassan YF, Eltukhi WE, Salman BI (2022) Ultra-Sensitive Fluorimetric Method for the First Estimation of Vonoprazan in Real Human Plasma and Content Uniformity Test. J Fluoresc 32:1725–1732. https://doi.org/10.1007/s10895-022-02979-2
Salman BI, Hassan AI, Hassan YF, Saraya RE (2022) Ultra-sensitive and selective fluorescence approach for estimation of elagolix in real human plasma and content uniformity using boron-doped carbon quantum dots. BMC Chem 16:58. https://doi.org/10.1186/s13065-022-00849-3
Hammad MA, Omar MA, Salman BI (2017) Utility of Hantzsch reaction for development of highly sensitive spectrofluorimetric method for determination of alfuzosin and terazosin in bulk, dosage forms and human plasma. Luminescence 32:1066–1071. https://doi.org/10.1002/bio.3292
Ali MFB, Salman BI, Hussein SA, Marzouq MA (2020) Applicability of ninhydrin as a fluorescent reagent for estimation of teicoplanin in human plasma using salting-out assisted liquid–liquid extraction technique. Luminescence 35:1118–1124. https://doi.org/10.1002/bio.3824
Huang H, Lv JJ, Zhou DL, Bao N, Xu Y, Wang AJ, Feng JJ (2013) One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv 3:21691–21696. https://doi.org/10.1039/c3ra43452d
Ghosh S, Gul AR, Park CY, Xu P, Baek SH, Bahmore JR, Kim MW, Lee M, Kialasa SK, Park TJ (2021) Green synthesis of carbon dots from Calotropis procera leaves for trace level identification of isoprothiolane. Microchem J 167:106272. https://doi.org/10.1016/j.microc.2021.106272
Jothi VK, Ganesan K, Natarajan A, Rajaram A (2021) Green Synthesis of Self-Passivated Fluorescent Carbon Dots Derived from Rice Bran for Degradation of Methylene Blue and Fluorescent Ink Applications. J Fluoresc 31:427–436. https://doi.org/10.1007/s10895-020-02652-6
Bae WK, Char K, Hur H, Lee S (2008) Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem Mater 20:531–539. https://doi.org/10.1021/cm070754d
Yang W, Yang H, Ding W et al (2016) High quantum yield ZnO quantum dots synthesizing via an ultrasonication microreactor method. Ultrason Sonochem 33:106–117. https://doi.org/10.1016/j.ultsonch.2016.04.020
Branch SK (2005) Guidelines from the International Conference on Harmonisation (ICH). J Pharm Biomed Anal 38:798–805. https://doi.org/10.1016/j.jpba.2005.02.037
Zimmer D (2014) New US FDA draft guidance on bioanalytical method validation versus current FDA and EMA guidelines: chromatographic methods and ISR. Bioanalysis 6:13–19. https://doi.org/10.4155/bio.13.298
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM (2020) Development of dual function polyamine-functionalized carbon dots derived from one step green synthesis for quantitation of Cu2+ and S2− ions in complicated matrices with high selectivity. Anal Bioanal Chem 412:1353–1363. https://doi.org/10.1007/s00216-019-02362-4
Wong YN, King SP, Laughton WB et al (1998) Single-Dose Pharmacokinetics of Modafinil and Methylphenidate Given Alone or in Combination in Healthy Male Volunteers. J Clin Pharmacol 38:276–282. https://doi.org/10.1002/j.1552-4604.1998.tb04425.x
United States Pharmacopeial Convention (905) (2011) Uniformity of Dosage Units. Stage 6 Harmonization 3:4–6
Ibrahim AE, Saraya RE, Saleh H, Elhenawee M (2019) Development and validation of eco-friendly micellar-HPLC and HPTLC-densitometry methods for the simultaneous determination of paritaprevir, ritonavir and ombitasvir in pharmaceutical dosage forms. Heliyon 5:e01518. https://doi.org/10.1016/j.heliyon.2019.e01518
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness Metric Approach and Software. Anal Chem 92:10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
Saraya RE, el Deeb S, Salman BI, Ibrahim AE (2022) Highly sensitive high-performance thin-layer chromatography method for the simultaneous determination of molnupiravir, favipiravir, and ritonavir in pure forms and pharmaceutical formulations. J Sep Sci 45:2582–2590. https://doi.org/10.1002/jssc.202200178
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Baher I. Salman designed the research, carried out experiments, interpreted the results and wrote the main manuscript. Ahmed I. Hassan designed the research, supervised the findings of the research. Yasser F. Hassan contributed to material preparation, data collection and analysis. Roshdy E. Saraya interpreted the results and tables analysis. Hany A. Batakoushy analyzed the data, draw the figures, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Plasma samples were collected and carried out according to (ethical standards of the responsible committee on human experimentation) (institutional and national) and with Helsinki Declaration of 1975, as revised in 2008.
Consent to Participate
The human experimental carried out at human healthy volunteers according to Helsinki Declaration of 1975, as revised in 2008.
Consent to publish
Not applicable.
Conflicts of Interest
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salman, B.I., Hassan, A.I., Hassan, Y.F. et al. Rapid One-Pot Microwave Assisted Green Synthesis Nitrogen Doped Carbon Quantum Dots as Fluorescent Precursor for Estimation of Modafinil as Post-Covid Neurological Drug in Human Plasma with Greenness Assessments. J Fluoresc 33, 1101–1110 (2023). https://doi.org/10.1007/s10895-022-03128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03128-5