Abstract
Sensitive and green spectrofluorimetric methods were utilized for Tamsulosin Hydrochloride (TAM) and Tadalafil (TDL) assessment in bulk and their newly available combined mixture for benign prostatic hyperplasia and erectile dysfunction. The technique relies on measuring native fluorescence of TAM in 0.1 N HCl at 324 nm and TDL in 0.1 N NaOH at 348 nm due to their different fluorimetric behavior in acidic and basic media where TAM has no fluorescence in basic medium and vice versa. To achieve better regression, the spectra were derivatized allowing determination of TAM at 314 nm and TDL at 320 and 380 nm (peak to peak) by applying third and first derivative, respectively. In addition, pH-dependent “constant-wavelength synchronous” spectrofluorimetry was applied where TAM and TDL were determined at 218 nm in acidic medium and at 268 nm in basic medium, respectively. Finally, derivatizing the latter emission spectra allowed determination of TAM and TDL at 232 nm and at 262 and 278 nm (peak to peak), respectively. Acidic and basic emission spectra where scanned at λexc = 225 nm (for TAM assay) and at λexc = 247 nm (for TDL assay), respectively. Fluorescence–concentration plots were linear and the proposed methods were used for analysis of TAM and TDL combined laboratory prepared formulation. These procedures are green, sensitive and of low cost which make them suitable for quality control analysis of the two drugs. In addition, the high selectivity of the proposed methods was tested by successfully applying them for TAM and TDL assay in plasma samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “Lower urinary tract symptoms, LUTS” refers to all voiding and obstructive urinary symptoms which are known to be strongly related to benign prostatic hyperplasia (BPH). LUTS and BPH are known to increase with age so they are widespread in elderly men, above 50 years of age [1]. Recently, several researches also linked the high occurrence of erectile dysfunction (ED) for patients with BPH [2,3,4].
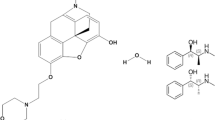
Tamsulosin HCl (TAM) is a selective α1A-adrenergic antagonist used as an effective treatment for BPH as it leads to relaxation of smooth muscle and urine obstruction relief [5]. TAM is chemically designed as 5-[(2R)-2-[[2-(2 Ethoxyphenoxy)ethyl]amino]-propyl]-2-methoxybenzenesulfonamide hydrochloride (Fig. 1a) [6, 7]. Tadalafil (TDL), a phosphodiesterase enzyme-5 inhibitor, is used originally to treat ED. But it has been recently approved to treat BPH patients with or without ED, where this can be attributed to its ability to reduce contraction of bladder neck and prostate and to improve urine flowage [8,9,10]. TDL is chemically known as (6R,12aR)-6-(1,3- Benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]-pyrido[3,4-b] indole-1,4-dione (Fig. 1b) [6, 7].
Recently, TAM and TDL have been formulated as a combined formulation for treating BPH associated with ED. This binary combination, in many researches, proved to be safe and more effective than using each drug separately [4, 11]. In addition, it enhances patient compliance by administrating only one fixed dose combination with lower side effects [4, 10].
Several methods in the literature are reported for TAM or TDL assay as single components or with other drugs such as spectrophotometry [12, 13], spectrofluorimetry [14, 15], electrochemistry [16, 17], HPTLC [18, 19] and HPLC [20, 21].
TAM and TDL specifically together were analyzed in the recent literature by spectrophotometry [22], HPTLC [23, 24] and HPLC [24]. The aim of this study was to utilize spectrofluorimetry as it is green, low cost and highly sensitive technique with low solvent consumption for TAM and TDL assay as a newly formulated binary mixture.
This study shows the multiplicity of fluorimetry in utilizing different spectrofluorimetric behavior of drugs in different pHs. Also, it put emphasizes on the sensitivity that can be achieved without breaching the rules of green analysis. The proposed spectrofluorimetric methods are dependent on the difference in relative fluorescence intensity (RFI) of TAM and TDL in acidic and basic media. Direct and derivative pH-dependent spectrofluorimetric and synchronous spectrofluorimetric measurements were applied and compared in terms of their sensitivity and selectivity. In addition, both validation and analysis of this binary combination in laboratory prepared tablets and spiked human plasma samples were fulfilled.
Experimental
Spectrofluorimetric Apparatus
Cary Eclipse Spectroflourimeter (G9800A, Agilent technologies) and quartz cells of 1 cm were used for all spectrofluorimetric measurements. It is also connected to a computer with Cary Eclipse software.
Reagents and Chemicals
-
TAM (99.99%) and TDL (100.06%) were brought from Sigma Aldrich, USA.
-
Methanol was HPLC grade (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland).
-
NaOH and HCl used in the study were Analar grade (El-Nasr Pharmaceutical Chemicals, Cairo, Egypt).
-
Human plasma sample was obtained from Alexandria blood bank (Alexandria, Egypt).
TAM and TDL newly marketed dosage form is unavailable in our commercial markets. Thus, laboratory-prepared tablets containing 0.4 and 5 mg of TAM and TDL, respectively, per tablet were used as their concentrations in the marketed tablets. The tablets were prepared using commonly used tablet fillers kindly supplied by Pharco Pharmaceuticals Co., Alexandria, Egypt, composed of maize starch, microcrystalline cellulose (avicel), magnesium sterate, hydroxyl propyl methyl cellulose and colloidal silica (aerosil).
Standard Solutions and Calibration Standards
Standard stock solutions of TAM and TDL were prepared in methanol, separately, with concentrations of 1000 μg/mL. These stock solutions were stable for a minimum of two weeks at 4 °C away from light. Aliquots (μLs, using micropipette) from TAM and TDL standard stock solutions (1000 μg/mL, each), corresponding to Table 1 linearity range, were transferred into two separate sets of 10-mL volumetric flasks and adjusted all to contain an overall of 200 μL methanolic content. TAM set was completed with 0.1 N HCl to the mark (for TAM determination) and the other TDL set was completed to mark with 0.1 N NaOH (for TDL determination).
Synthetic Mixtures
Aliquots of TAM and TDL were transferred from their corresponding standard stock solutions into a set of 10-mL volumetric flasks to achieve linearity ranges stated in Table 1. All volumetrics were adjusted to have a constant volume of methanol of 200 μL, by adding the appropriate volume of methanol on the drugs’ aliquots in each flask. The volumetric flasks were then completed with 0.1 N HCl to the mark (for TAM determination) one time and another time with 0.1 N NaOH (for TDL determination).
Applied Procedures
Construction of Calibration Curves
For each calibration standard of TAM and TDL prepared in “Standard Solutions and Calibration Standards” section, the emission spectra (1 nm interval) were recorded with excitation at 225 and 247 nm for acidic and basic solutions, respectively. All emission spectra were corrected with methanolic HCl or NaOH blank signal and processed using Microsoft Excel®.
Direct Fluorimetric Method
The amplitude values of the acidic emission spectra in 0.1 N HCl at 324 nm, were used for TAM determination (where TDL shows no RFI). Also, amplitude values of the alkaline emission spectra in 0.1 N NaOH at 348 nm were measured for the determination of TDL (where TAM shows negligible RFI).
Derivative Fluorimetric Method
The third derivative values of the acidic emission spectra were computed at 314 nm for TAM determination (TDL zero crossing) using Δλ of 3 nm. Also, the first derivative values of the alkaline emission spectra were computed by measuring peak to peak at 320 and 380 nm (TAM zero crossing) for the determination of TDL using Δλ of 3 nm.
Direct Synchronous Method
Synchronous spectra of both drugs in acidic and basic solvents were scanned in range of 190–400 nm keeping 90 nm constant difference between the two monochromators of excitation and emission. Values of the synchronous emission spectra in HCl at 218 nm, were used for TAM determination (where TDL shows negligible RFI). Also, values of the synchronous emission spectra in NaOH at 268 nm, were computed for determination of TDL.
Derivative Synchronous Method
The first derivative values of the acidic synchronous spectra were measured at 232 for the determination of TAM (TDL zero crossing) and in 0.1 N NaOH, peak to peak measurements were done at 262 and 278 nm for the determination of TDL, all using Δλ of 3 nm.
Analysis of Synthetic Mixtures
TAM and TDL mixtures with different ratios were measured against suitable blank and their emission spectra were computed by the four methods to assay the concentration of each drug and its recovery in each mixture.
Analysis of Laboratory Prepared Tablets
Ten laboratory-prepared tablets were weighed and powdered well and a weight equivalent to 2 and 25 mg TAM and TDL, respectively was transferred by methanol, into a 25 mL volumetric flask. After sonication for 15 min, dilution was made with methanol to volume and filtered using Whatman No-1 filter paper. A 125 μL portion of the prepared sample solution and 75 μL methanol were added into two 10-mL volumetric flasks, then one of them was diluted to volume with 0.1 N HCl and the other with 0.1 N NaOH. The concentrations achieved were 1 and 12.5 μg/mL of TAM and TDL, respectively. The procedures were completed on the prepared solutions as previously described.
Analysis of Spiked Human Plasma Samples
Aliquots from TAM and TDL methanolic stock solutions were added to 1 mL plasma samples to prepare five mixtures with different ratios (1: 12.5, 1: 6, 1: 1, 10: 1, and 1: 5 for TAM: TDL). The solutions were vortexed for 3 min after being completed to a total volume of methanol = 5 mL and the tubes were then centrifuged at 4000 rpm (15 min). A 200 μL of the supernatant was carefully transferred into 10 mL volumetric flasks and completed to volume with either HCl or NaOH where the analysis was continued as described previously against blanks.
Result and Discussion
TAM and TDL are recently co-formulated together in a newly marketed dosage form where TAM is the minor component with concentration of 0.4 mg in comparison to TDL (5 mg). In pharmaceutical industry and quality control analysis, the development of new assay techniques to analyze newly challenging co-formulated and marketed drug products should always be addressed with its challenges, due to its importance.
Usually analysts resolve multi component mixtures using chromatographic methods as HPLC [25] and HPTLC [26] using organic toxic solvents with large amounts. However, spectrofluorimetry is a sensitive, green and low cost method that is highly favored in pharmaceutical analysis of complex mixtures. For strong spectral overlaps, derivative spectrofluorimetry is usually applied allowing simultaneous determination of complex mixtures as it improves minor spectral characteristics [12, 14, 27, 28].
Meanwhile, in constant-wavelength synchronous fluorimetry: the emission spectra are measured by synchronously scanning excitation and emission monochromators with a constant ∆λ offering much higher spectral resolution, selectivity and sensitivity because it gives sharp and narrow spectra.
Upon preliminary scanning of TAM and TDL emission spectra (Fig. 2), it was noticed the severe similarity in the fluorimetric spectra and behavior of TAM and TDL in several solvents as methanol, water and acetonitrile which hinders their simultaneous determination even after mathematical transformations as derivative or synchronous or Fourier convolution. In addition, TAM being the minor component in the pharmaceutical dosage form ratio, its spectrofluorimetric spectrum was completely covered by that of TDL which is very typical to it.
By noticing the spectral behavior of the two concerned drugs in several solvents (Fig. 3), it was noticed that TAM had shown acceptable RFI in acidic medium using 0.1 N HCl and no RFI in alkaline medium using 0.1 N NaOH and TDL had opposite behavior. Thus, the proposed spectrofluorimetric methods took use of the different spectrofluorimetric behavior of both drugs in different pH and resolved there strong overlapping without preliminary separation, or tedious mathematical calculations. This was achieved by simply measuring TAM in acidic medium and TDL in basic medium in presence of each other without interference and with satisfactory selectivity, accuracy and precision, even in plasma samples.
Method Development and Optimization
Direct Fluorimetric Method
TAM and TDL presents very similar native fluorescence. Several solvents were tried but the only difference between the emission spectra of both drugs was in acidic and basic media upon excitation at their corresponding λex (225 nm for TAM acidic spectra and 247 nm for TDL basic spectra) as explained earlier and shown in Fig. 3. The acidic emission spectrum of TAM was measured at 324 nm where TDL (major component) had zero-crossing. Meanwhile, the basic emission spectrum of TDL was measured at 348 nm for TDL determination (TAM has no RFI in basic medium) (Fig. 4a, b).
Emission spectra of 2.5 μg/mL TAM, 31.25 μg/mL TDL and their mixture in (a) 0.1 N HCl (λexc = 225 nm), (b) 0.1 N NaOH (λexc = 247 nm), (c) 0.1 N HCl (D3 using Δλ = 3 nm) and (d) 0.1 N NaOH (D1 using Δλ = 3 nm) and constant wavelength synchronous spectra (Δλ = 90 nm) of 1 μg/mL TAM, 12.5 μg/mL TDL and their mixture in (e) 0.1 N HCl, (f) 0.1 N NaOH, (g) 0.1 N HCl (D1 using Δλ = 3 nm) and (h) 0.1 N NaOH (D1 using Δλ = 3 nm). (TAM
 , TDL
, TDL
 and MIX
and MIX
 )
)
Derivative Fluorimetric Method
Derivative fluorimetry is known to improve resolution of overlapping bands and increase compounds’ spectral characteristics [12, 14, 27, 28]. In case of TAM and TDL binary mixture, the resulted acidic spectra after third derivative revealed that TAM could be determined by peak measurement at 314 nm without any interference from TDL, as the latter showed zero crossing. On the other hand, TDL could be analyzed by peak to peak measurements at 320 and 380 nm with zero crossing of TAM at these two wavelengths using first derivative of the alkaline spectra (Fig. 4c, d).
For choosing best wavelength interval (Δλ) for TAM and TDL assay, different Δλs were tried from 3–11 nm to derivatize the data. The Δλ giving best linearity, sensitivity and percentage recoveries was Δλ of 3 nm.
Direct Synchronous Method
Synchronous “constant-wavelength” mode was also tried to improve spectral resolution and allow measurement of the two drugs directly without mathematical derivatization where the two monochromators are simultaneously scanned with constant Δλ. Several Δλs were studied from 10 to 100 nm. For obtaining the synchronous spectra of these two drugs with maximum spectral resolution and sensitivity for TAM (the minor component), Δλ = 90 nm was found suitable and gave reasonable response for both drugs at their dosage form ratio.
Synchronous emission spectra had advantages over conventional spectrofluorimetry in giving much simpler narrow spectra, allowing quantitative determinations with higher sensitivity than the first two methods (Table 1). However, applying synchronous measurements on methanolic solutions of both drugs revealed highly overlaid spectra. Thus, synchronous technique was applied also on solutions of both drugs in basic and acidic media to create a difference in their fluorimetric behavior. The emission values at 218 nm in acidic medium and at 268 nm in basic medium were used for the determination of TAM and TDL, respectively, in their mixture (Fig. 4e, f).
Derivative Synchronous Method
The first derivative values of the synchronous spectra in HCl were measured at 232 nm for the determination of TAM and in NaOH, peak to peak measurements were done at 262 and 278 nm for the determination of TDL, all using Δλ of 3 nm. (Fig. 4g, h).
The selected wavelengths for each drug showed zero contribution from the other component in the mixture allowing its accurate determination. The optimization of Δλ was also studied in the same range (3–11 nm) and Δλ = 3 nm was also chosen.
Effect of pH
The pH impact on TAM and TDL fluorescence was studied using various types of buffers such as 0.2 M acetate buffer (pH 3.5–6) and 0.2 M borate buffer (pH 6.5–8.5), in addition to 0.1 N HCl and 0.1 N NaOH. The buffer usage had negative impact on the RFI over the entire studied pH range. It was found that maximum RFI was obtained only in 0.1 N HCl for TAM and 0.1 N NaOH for TDL (Fig. 5).
Validation
The methods were validated according to ICH guidelines [29]:
Linearity and Sensitivity
Linearity was checked for all the proposed methods within the ranges stated in Table 1 including their dosage form ratio (1:12.5, TAM: TDL). Statistical assessment of the results shows acceptable correlation coefficient (r) values, small values of standard deviation of residuals (Sy/x), standard deviation of intercept (Sa) and standard deviation of slope (Sb), in addition to high F-values with low significance F-values (Table 1). LOD and LOQ were calculated according to Miller: LOD = 3.3Sa/b and LOQ = 10Sa/b [30]. Derivatizing the emission spectra improved the regression and correlation coefficients of the data (increase in r values). Also, the synchronous methods showed better sensitivity and lower LOD and LOQ. The proposed methods are spectrofluorimetric methods for analysis of TAM and TDL with much better sensitivity compared with spectrophotometric methods. Also, they are superior to chromatographic methods in having simpler steps, less time, lower cost and less solvent consumption making them green and eco-friendly.
Accuracy, Precision and Selectivity
Acceptable accuracy and precision were indicated by the % recoveries (± 2%) and low RSD% values (≤ 2%) after analysis of synthetic mixtures of TAM and TDL at different ratios as stated in Table 2. The samples were processed on one day and on three different days (n = 3). Successful selective determination of TAM and TDL with no interference from each other, in different ratios, also shows the selectivity of the proposed methods.
Solutions Stability
The prepared solutions of TAM and TDL were found to be stable when left at room temperature for at least 6 h which was sufficient for all measurements (RSD% ≤ 2%).
Assay of Laboratory Prepared Tablets
The proposed methods were applied for TAM and TDL analysis in laboratory prepared tablets without interference. The obtained recoveries of the studied drugs were in agreement with the label claims (Table 3). A comparison between the proposed method and another reported spectroscopic method was done by Student’s t- and variance ratio F- tests. Calculated values did not exceed theoretical ones (at 95% confidence level) which prove there is no significant difference between the developed fluorimetric methods and the comparison method (Table 3).
Analysis of TAM and TDL in Spiked Human Plasma Samples
TAM and TDL assay in spiked plasma samples was performed in different ratios to test methods’ selectivity with just simple protein precipitation step. As shown in Table 4, high % recoveries (95–105%) and low % RSD values (≤ 2%) proved the ability and high selectivity of these fluorimetric methods to assay TAM and TDL in spiked human plasma with minimal solvent consumption and cost. (Table 4).
Conclusion
New analytical methods must be available for analysis of newly marketed pharmaceutical combinations for treating different conditions. This is the case for TAM and TDL recently developed co-formulation for treating benign prostate hyperplasia and erectile dysfunction in elderly men. Four simple, selective and time saving fluorimetric methods were established for resolving this mixture in its challenging ratio. Green analytical chemistry is based on minimal use of expensive and toxic solvents and instruments consuming large amount of energy (like HPLC). The proposed fluorimetric methods fulfilled the criteria of green chemistry as it only used simple solvents as HCl and NaOH with the spectrofluorimeter. Derivatizing the spectra in general improved the data’s regression and synchronizing the spectra with constant wavelength difference changed the characteristics of the emission spectra which allowed more sensitive determination of the two drugs. The proposed methods could analyse the two drugs in their pure drugs substances, their combined laboratory made dosage form and spiked human plasma samples.
Availability of Data and Materials
The datasets used and/or analysed during the current study are all available in the manuscript in details, for more information contact the corresponding author.
References
Dutkiewicz S, Skawiński D, Duda W, Duda M (2012) Assessing the influence of benign prostatic hyperplasia (BPH) on erectile dysfunction (ED) among patients in Poland. Cent European J Urol 65:135–138. https://doi.org/10.5173/ceju.2012.03.art7
Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs R, Fourcade R, Kiemeney L, Lee C, UrEpik Study Group (2003) The association between lower urinary tract symptoms and erectile dysfunction in four centres: the UrEpik study. BJU Int 92:719–725. https://doi.org/10.1046/j.1464-410X.2003.04459.x
Glina S, Glina FPA (2013) Pathogenic mechanisms linking benign prostatic hyperplasia, lower urinary tract symptoms and erectile dysfunction. Ther Adv Urol 5:211–218. https://doi.org/10.1177/1756287213488236
Kim SW, Park NC, Lee SW, Yang DY, Park JK, Moon DG et al (2017) Efficacy and safety of a fixed-dose combination therapy of tamsulosin and tadalafil for patients with lower urinary tract symptoms and erectile dysfunction: results of a randomized, double-blinded, active-controlled trial. J Sex Med 14:1018–1027. https://doi.org/10.1016/j.jsxm.2017.06.006
Committee APG (2003) AUA guideline on management of benign prostatic hyperplasia. Chapter 1: Diagnosis and treatment recommendations. J Urol 170:530–547
British Pharmacopeia (2019)The Stationary Office, volume II, London
US Pharmacopeia 33 (2011) The united states pharmacopial convention —The National Formulary, 28th ed. United States Pharmacopeial Convention, Inc, USA
Kim EH, Larson JA, Andriole GL (2016) Management of benign prostatic hyperplasia. Annu Rev Med 67:137–151. https://doi.org/10.1146/annurev-med-063014-123902
EAU Guidelines Edn (2019) Presented at the EAU Annual Congress Barcelona. ISBN 978–94–92671–04–2
Furuta A, Suzuki Y, Igarashi T, Koike Y, Egawa S, Yoshimura N (2018) Effects of combined treatment of tadalafil and tamsulosin on bladder dysfunction via the inhibition of afferent nerve activities in a rat model of bladder outlet obstruction. Int Urol Nephrol 50:839–844. https://doi.org/10.1007/s11255-018-1835-8
Karami H, Hassanzadeh-Hadad A, Fallah-Karkan M (2016) Comparing monotherapy with tadalafil or tamsulosin and their combination therapy in men with benign prostatic hyperplasia: a randomized clinical trial. Urol J 13:2920–2926
El-Kimary E, Khamis E, Belal S, Moneim MA (2020) Fourier convolution versus derivative spectrophotometry: Application to the analysis of two binary mixtures containing tamsulosin hydrochloride as a minor component. Ann Pharm Fr 78:129–141. https://doi.org/10.1016/j.pharma.2020.01.003
Khan ZG, Patil AS, Shirkhedkar AA (2014) Estimation of tadalafil using derivative spectrophotometry in bulk material and in pharmaceutical formulation. Int J Spectrosc. https://doi.org/10.1155/2014/392421
El-Kimary EI, Khamis EF, Belal SF, Abdel Moneim MM (2018) Enhanced spectrofluorimetric determination of two novel combination therapies for the treatment of benign prostatic hyperplasia containing tamsulosin hydrochloride. J Lumin 33:771–779. https://doi.org/10.1002/bio.3475
Abu El-Enin MAB, Al-Ghaffar Hammouda MESA, El-Sherbiny DT, El-Wasseef DR, El-Ashry SM (2016) Validated spectrofluorimetric method for determination of two phosphodiesterase inhibitors tadalafil and vardenafil in pharmaceutical preparations and spiked human plasma. J Lumin 31:173–178. https://doi.org/10.1002/bio.2941
Lonappan L, Issac S, Joseph R, Thomas D, Kumar KG (2011) Electrochemical studies of tamsulosin hydrochloride using multiwalled carbon nanotube-modified glassy carbon sensor. Micro Nano Lett 6:867–870. https://doi.org/10.1049/mnl.2011.0384
Sartori ER, Clausen DN, Pires IMR, Salamanca-Neto CAR (2017) Sensitive square-wave voltammetric determination of tadalafil (Cialis®) in pharmaceutical samples using a cathodically pretreated boron-doped diamond electrode. Diam Relat Mater 77:153–158. https://doi.org/10.1016/j.diamond.2017.07.001
El-Kimary EI, Khamis EF, Belal SF, Abdel Moneim MM (2018) Novel validated HPTLC method for the analysis of two binary mixtures containing tamsulosin hydrochloride with antimuscarinic agents. J Chromatogr Sci 56:81–91. https://doi.org/10.1093/chromsci/bmx081
Naguib IA, Magdy MA, Anwar BH, Abdelhamid NS (2020) A validated green HPTLC method for quantitative determination of dapoxetine hydrochloride and tadalafil in bulk and pharmaceutical formulations. J Chromatogr Sci 58:303–308. https://doi.org/10.1093/chromsci/bmz115
Ganthi HKR, Reddy R, Park YJ, Bapatu HR, Park SJ, Cho WH (2016) Stability indicating HPLC method for quantification of solifenacin succinate & tamsulosin hydrochloride along with its impurities in tablet dosage form. Am J Analyt Chem 7:840–862. https://doi.org/10.4236/ajac.2016.711073
Raul SK, Kumar B, Pattnaik AK (2012) A RP-HPLC Method Development and Validation for the Estimation of Tadalafil in Bulk and Pharmaceutical Dosage Forms. Res J Pharm Technol 5:1573–1576
Rezk MR, Tantawy MA, Wadie M, Weshahy SA (2020) Smart spectrophotometric assessment of tamsulosin hydrochloride and tadalafil in their new pharmaceutical formulation for treatment of benign prostatic hyperplasia and erectile dysfunction. Spectrochim Acta A Mol Biomol Spectrosc 227:117547. https://doi.org/10.1016/j.saa.2019.117547
Tantawy MA, Weshahy SA, Wadie M, Rezk MR (2020) Novel HPTLC densitometric methods for determination of tamsulosin HCl and tadalafil in their newly formulated dosage form: Comparative study and green profile assessment. Biomed Chromatogr 34(8):e4850. https://doi.org/10.1002/bmc.4850
Rezk MR, Abdel-Moety EM, Wadie M, Tantawy MA (2021) Stability assessment of tamsulosin and tadalafil co-formulated in capsules by two validated chromatographic methods. J Sep Sci 44:530–538. https://doi.org/10.1002/jssc.202000975
Belal FF, Sharaf El-Din MK, Tolba MM, Elmansi H (2015) Determination of two ternary mixtures for migraine treatment using HPLC method with ultra violet detection. Sep Sci Technol 50:592–603. https://doi.org/10.1080/01496395.2014.957317
Youssef R, Khamis E, El-Sayed M, Abdel Moneim M (2014) Development and validation of a high-performance thin-layer chromatographic method for the assay of ternary mixtures containing cetirizine dihydrochloride in pharmaceutical dosage forms. JPC J Planar Chromat-Mod TLC 27:58–65. https://doi.org/10.1556/JPC.27.2014.1.11
Eman I, Ragab MA (2018) Derivative synchronous spectrofluorimetry: application to the analysis of two binary mixtures containing codeine in dosage forms. Spectrochim Acta A Mol Biomol Spectrosc 204:677–684. https://doi.org/10.1016/j.saa.2018.06.102
Abdel Moneim MM, Hamdy MMA (2021) Green spectrofluorimetric methods for tramadol assay with ibuprofen or chlorzoxazone: comparison of greenness profiles. Luminescence 36:497–505. https://doi.org/10.1002/bio.3969
International Conference on Harmonization (2005) Validation of analytical procedures. Text and methodology, Q2A. Geneva: R1
Miller J, Miller JC (2018) Statistics and chemometrics for analytical chemistry: Pearson education
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The author is responsible for conceptualization, practical work, methodology, data analysis, manuscript 1st draft writing and reviewing and editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not Applicable
Consent for Publication
Not Applicable
Competing Interests
The author declares that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel Moneim, M.M. Green & Sensitive pH-dependent Spectrofluorimetric Assay of Tamsulosin Hydrochloride and Tadalafil in their New Combined Formulation for Benign Prostatic Hyperplasia: Application to Spiked Human Plasma. J Fluoresc 32, 1581–1590 (2022). https://doi.org/10.1007/s10895-022-02938-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02938-x